Abstract
Rationale and objectives
A new microcatheter was recently developed claiming to reduce beads reflux in drug-eluting bead transarterial chemoembolization (DEB-TACE). The aim of this study was to compare the reflux control microcatheter ability versus a standard microcatheter for TACE treatment in patients with hepatocellular carcinoma.
Material and methods
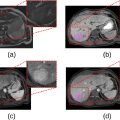
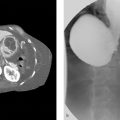
Patients were prospectively included between November 2017 and February 2022. They received a DEB-TACE treatment with charged radiopaque beads using standard microcatheters or the SeQure reflux control microcatheter (Guerbet, France) and were assigned respectively to a control and a test group. Beads distribution mismatch was evaluated between the targeted territory on treatment planning CBCT and beads’ spontaneous opacities on the post-intervention CBCT and the 1-month CT scanner.
Results
Twenty-three patients (21 men, median age 64 years [12.5 years]) with 37 hepatocellular carcinoma nodules were treated. The control group consisted of 13 patients – 19 nodules, while the test group included ten patients – 18 nodules. Non target embolization (NTE) was found in 20 % (2/10) of patients in the test group and 85 % (11/13) in the control group. NTE involved only an adjacent segment in the test group while it affected the adjacent biliary sector or even the contralateral liver lobe in the control group. No complication linked to NTE was found in the test group, while it led to one case of ischemic cholangitis and another case of biloma in the control group.
Conclusion
The reflux control microcatheter may be efficient in reducing NTE and thus eventual adverse events in comparison to standard of care end-hole microcatheters.
Graphical abstract

1
Introduction
Liver cancer is the third most frequent cause of cancer-related death around the globe. Hepatocellular carcinoma (HCC) is by far the most common type of primary liver cancers (75 to 85 %) , and the most common risk factor is liver cirrhosis.
Transarterial chemoembolization (TACE) is the recommended treatment for intermediate stage HCC when curative options are not available. In conventional TACE (cTACE), the chemotherapeutic agent, most frequently doxorubicin, is injected in an emulsion with Lipiodol® (Guerbet, Villepinte, France) into the branch of the hepatic artery feeding the tumor , followed by the injection of an embolic material . In a more recent TACE technique, called drug-eluting bead TACE (DEB-TACE) , calibrated beads are loaded with chemotherapeutics beforehand, so as to provide a more consistent embolization and a more controlled and sustained delivery of the drug.
Antireflux or flow directed microcatheters have been developed and marketed since a few years, aiming to “avoid” non target embolization and increase drug and bead delivery to the target lesion [ , ].Those devices rely on mechanically occluding the feeding artery through most commonly a distal tip balloon-occlusion microcatheter, thus preventing reflux and increasing beads penetration into the target lesion due to reduced downstream blood pressure following the obstruction. Their use is however debated due to cases of iatrogenic arterial wall injury [ , ] and potential opening of interlobar and intersegmental vascular shunts [ , ].A new reflux control microcatheter (SeQure Guerbet company, France) has recently been developed. It is different from traditional antireflux microcatheters with a view to preventing beads reflux by creating a fluid barrier of turbulent flow at the distal end of the microcatheter through microscopic side holes , without any occluding device or obstruction of the natural arterial flow. It has been tested in vitro and in vivo using regular, radiolucent beads and obtained FDA approval in January 2018 and CE mark approval in April 2019 .
However, most beads are radiolucent and have to be mixed with contrast media allowing only an indirect visualization of the embolic material, making postintervention target evaluation and beads distribution rather difficult to distinguish from contrast trapping . In the past years, radiopaque beads have been developed to provide intraprocedural feedback on treated locations, to help avoid nontarget embolization, and to better evaluate tumor embolization [ , ].
The main objective of this study was to assess the effectiveness of the SeQure microcatheter by using it with radiopaque beads to evaluate its reflux control properties compared with a standard-of-care catheter for nontarget embolization during DEB-TACE procedures. Secondary objectives were the evaluation of tumor coverage by the embolic agents during and following the procedure, as well as tumor response and adverse events.
2
Methods
2.1
Patients
We prospectively included all patients with focal or multifocal HCC, either primary or recurrent, who were candidates for a DEB-TACE treatment between November 2017 and February 2022 in our department. The institutional review board approval was obtained (IRB approval number: CRM-2206–278). All the procedures were performed in compliance of the ethical standards adopted by our institution’s research and ethics committee, the 1964 Helsinki declaration and its subsequent amendments. The study was assessed using STROBE (STrengthening the Reporting of OBservationalv studies in Epidemiology) guidelines . The patients were selected for TACE treatment after their consent and following multidisciplinary consensus, in accordance with current established guidelines for the management of HCC as mentioned below. The multidisciplinary tumor board included oncology, hepatology, visceral and digestive surgery, radiation oncology, nuclear medicine, pathology, and diagnostic radiology specialists. TACE treatment was then planned during an interventional radiology staff meeting. Criteria for receiving TACE treatment included HCC, absence of extrahepatic spread, absence of complete portal occlusion, Eastern Cooperative Oncology Group (ECOG) performance status ≤ 1, and Child-Pugh class A or B7, all in accordance to the Barcelona Clinic Liver Cancer (BCLC) guidelines , intermediate stage of the European Association for the Study of Liver (EASL) classification and the European Society of Medical Oncology (ESMO) guidelines . Patients were excluded if they had undergone prior TACE treatment with radiopaque material of the same nodule or segment.
The choice of DEB-TACE over cTACE was made during the interventional radiology staff meeting for the sole purpose of this study as the main chemoembolization intervention at our institution is cTACE. Patients were eligible for DEB-TACE if superselective catheterization (subsegmental or beyond) of the feeding artery was deemed possible as the radiopaque beads (DC Bead LUMI™, Boston Scientific, Massachusetts USA) used in this technique measure 70 to 150 μm and thus convey a higher risk of biliary complications [ , ]. All their medical records were reviewed and analyzed.
Three hundred and eighty-two patients received a TACE treatment at our institution during the inclusion period.
Among them, only 23 patients had a DEB-TACE and were included in the study ( Fig. 1 ), 21 (21/23, 96 %) of whom were male. The median age was 64 years old (25th percentile Q1: 62 years, 75th percentile Q3: 74 years). All but one had comorbidities including hypertension (61 %); type 2 diabetes (44 %); past or current tobacco smoking (59 %); cardiopathy (22 %); and a history of another neoplasm (26 %).

Twenty-one patients (96 %) had underlying liver cirrhosis, most frequently multifactorial, and 16 of these patients (74 %) had a history of alcohol-related liver disease.
The HCC was unifocal in ten patients (44 %) and de novo in 17 (74 %). The Child-Pugh score was A in 14 patients (61 %), and B7 in nine (39 %). Treatment by TACE was recommended for 12 patients (52 %) who were classified as BCLC B, and for ten patients (44 %) with BCLC A and one patient (4 %) with BCLC 0 as a bridge to liver transplantation.
Five patients (21 %) had undergone prior cTACE, six patients (25 %) a prior surgical resection, four (17 %) had percutaneous thermal ablation, all in a different liver segment from the nodule treated. One patient (4 %) had received previous systemic chemotherapy.
The patients were consecutively and alternatively assigned into two groups based on the microcatheter used: the test group for the SeQure microcatheter, and the control group for standard-of-care, end-hole, microcatheter.
The test group was composed of ten patients with 18 nodules, and the control group of 13 patients with 19 nodules ( Fig. 1 ). Three additional patients were added in the control group to reach an equivalent number of treated nodules in each group.
Patient demographics and characteristics are detailed in Table 1 .
| Total ( n = 23) | Control group ( n = 13) | Test group ( n = 10) | p | |
|---|---|---|---|---|
| Sex n (ratio,%) | 0.21 | |||
| Male | 21 (21/23; 91 %) | 11 (11/13; 85 %) | 10 (10/10; 100 %) | |
| Female | 2 (2/23, 9 %) | 2 (2/13; 15 %) | 0 | |
| Age years | (64; 62, 74) | 63; 62, 67 | 70; 61, 75 | 0.46 |
| Body m ass i ndex (mean ± SD), kg/m² | (28; 24, 33) | 26; 24, 31 | 30; 25, 34 | 0.29 |
| ECOG n (ratio,%) | 0.37 | |||
| 0 | 14 (14/23; 61 %) | 9 (9/13, 69 %) | 5 (5/10, 50 %) | |
| 1 | 9 (9/23; 39 %) | 4 (4/13, 31 %) | 5, (5/10, 50 %) | |
| ≥ 2 | 0 (0 %) | |||
| Comorbidities n (ratio,%) | ||||
| Hypertension | 14 (14/23; 61 %) | 9 (9/13, 69 %) | 5 (5/10, 50 %) | 0.37 |
| Type 2 diabetes | 10 (10/23; 44 %) | 4 (4/13, 31 %) | 6 (6/10, 60 %) | 0.18 |
| Tobacco smoking | 13 (13/23; 59 %) | 8 (8/13, 62 %) | 5 (5/10, 50 %) | 0.6 |
| Cardiopathy | 5 (5/23; 22 %) | 2 (2/13; 15 %) | 3 (3/10, 30 %) | 0.42 |
| Other cancers | 6 (6/23; 26 %) | 3 (3/13, 23 %) | 3 (3/10, 30 %) | 0.72 |
| Liver cirrhosis, n (ratio,%) | 22 (22/23; 96 %) | 12 (12/13, 92 %) | 10 (10/10, 100 %) | 0.39 |
| Cause of liver cirrhosis n (ratio,%) | ||||
| Alcohol-related liver disease | 17 (17/23; 74 %) | 8 (8/13, 62 %) | 9 (9/10, 90 %) | 0.13 |
| Non-alcoholic steatohepatitis | 10 (10/23; 44 %) | 5 (5/13, 38 %) | 5 (5/10, 50 %) | 0.6 |
| Viral hepatitis | 6 (6/23; 26 %) | 5 (5/13, 38 %) | 1 (1/10, 10 %) | 0.13 |
| Primary biliary cholangitis | 1 (1/23; 4 %) | 1 (1/13, 8 %) | 0 | 0.39 |
| Characteristics of HCC n (ratio,%) | ||||
| Unilocular | 10 (10/23; 44 %) | 8 (8/13, 62 %) | 2 (2/10, 20 %) | 0.05 |
| Multifocal | 13 (13/23; 57 %) | 5 (5/13, 38 %) | 8 (8/10, 80 %) | 0.05 |
| De novo | 17 (17/23; 74 %) | 9 (9/13, 69 %) | 8 (8/10, 80 %) | 0.6 |
| Recurrent | 6 (6/23; 26 %) | 4 (4/13, 31 %) | 2 (2/10, 20 %) | 0.6 |
| BCLC n (ratio,%) | ||||
| 0 | 1 (1/23; 4 %) | 1 (1/13, 8 %) | 0 | 0.39 |
| A | 10 (10/23; 44 %) | 6 (6/13, 46 %) | 4 (4/10, 40 %) | 0.78 |
| B | 12 (12/23; 52 %) | 6 (6/13, 46 %) | 6 (6/10, 60 %) | 0.53 |
| C | 0 (0/23; 0 %) | 0 | 0 | N/A |
| Child-Pugh n (ratio,%) | ||||
| A | 14 (14/23, 61 %) | 9 (9/13, 69 %) | 5 (5/10, 50 %) | 0.37 |
| B | 9 (9/23; 39 %) | 4 (4/13, 31 %) | 5 (5/10, 50 %) | 0.37 |
| C | 0 (0 %) | 0 | 0 | N/A |
| MELD | (11; 9, 15) | 9; 7, 11 | 14; 11, 16 | 0.03 |
| Prior treatment n (ratio,%) | ||||
| TACE | 5 (5/23; 21 %) | 3 (3/13, 23 %) | 2 (2/10, 20 %) | 0.78 |
| Surgical resection | 6 (6/23; 25 %) | 4 (4/13, 31 %) | 2 (2/10, 20 %) | 0.5 |
| Thermal ablation | 4 (4/23; 17 %) | 1 (1/13, 8 %) | 3 (3/10, 30 %) | 0.22 |
| Chemotherapy | 1 (1/23; 4 %) | 1 (1/13, 8 %) | 0 | 0.37 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree