Extraskeletal Osseous and Cartilaginous Lesions
The radiologic images of extraskeletal osseous and cartilaginous tumors of the extremities are often sufficiently characteristic to suggest a specific diagnosis. This is particularly true with regard to myositis ossificans and fibrodysplasia ossificans progressiva, as well as selected instances of soft tissue chondroma and extraskeletal osteosarcoma and chondrosarcoma.
The World Health Organization (WHO) currently classifies only soft tissue chondroma, extraskeletal mesenchymal chondrosarcoma and extraskeletal osteosarcoma as chondro-osseous tumors (1). Myositis ossificans and fibro-osseous pseudotumor of the digits are currently classified as fibroblastic/myofibroblastic tumors. Extraskeletal myxoid chondrosarcoma is classified as a tumor of uncertain differentiation. Rather than limit this chapter to those lesions strictly defined as chondroosseous tumors, we have chosen to demonstrate the radiologic spectrum of extraskeletal osseous and cartilaginous tumors and tumor-like conditions. Such lesions are not uncommon in clinical practice, and although it is useful to group these lesions together for purposes of differential diagnosis, it is worthwhile to remember that they have diverse origins.
With the exception of bizarre parosteal osteochondromatous proliferation (BPOP), lesions of the periosteum and juxtacortical regions are not considered in this chapter. Several soft tissue tumors, such as lipoma, liposarcoma, peripheral nerve sheath tumor, and so on, may contain metaplastic bone and/or cartilage; however, they are not considered to be primary osseous or cartilaginous tumors, and are discussed in their respective sections.
BENIGN LESIONS
Osseous Lesions
Benign osseous lesions are relatively uncommon, accounting for less than 1% of all benign soft tissue masses undergoing biopsy (2). The actual prevalence is difficult to ascertain with certainty, because the radiographic features are often characteristic, and many lesions are not excised or biopsied. The most common benign bone-forming lesion is myositis ossificans. Variants of this lesion located in the subcutis are termed panniculitis ossificans, and those localized to the fascia are referred to as fasciitis ossificans (3, 4). When the lesion arises in the periosteum, it is typically located in the hand (and less commonly in the foot), and is known as florid reactive periostitis of the tubular bones of the hands and feet or fibro-osseous pseudotumor of the digits (4).
KEY CONCEPTS
Myositis ossificans is the most common benign bone forming lesion.
It is termed panniculitis ossificans when it arises in the subcutis.
It is termed fasciitis ossificans when it arises in the fascia.
It is termed florid reactive periostitis or fibro-osseous pseudotumor when it arises in the periosteum.
Other benign osseous lesions include fibrodysplasia ossificans progressiva, soft tissue aneurysmal bone cyst, soft tissue osteoma, and bizarre parosteal osteochondromatous proliferation (BPOP).
Additional rare, benign, soft tissue lesions that will be discussed in this chapter include soft tissue fibrodysplasia ossificans progressiva, previously termed myositis ossificans progressiva, soft tissue aneurysmal bone cyst, soft tissue osteoma, and BPOP.
Myositis Ossificans
Myositis ossificans is a benign, solitary, self-limiting, ossifying, soft tissue mass typically occurring within skeletal muscle. A history of trauma is often absent; no distinction is made between lesions of atraumatic and traumatic origin. The pathogenesis of myositis ossificans is unknown and the term myositis is a misnomer because no primary inflammation of skeletal muscle is associated with the process (4, 5). Synonyms include pseudomalignant osseous tumor of soft tissue, extraosseous localized nonneoplastic bone and cartilage formation, myositis ossificans circumscripta, myositis ossificans traumatica, pseudomalignant myositis ossificans, and heterotopic ossification (5, 6, 7, 8).
The most frequent symptoms are pain, tenderness, and a soft tissue mass; however, the lesion may be an incidental finding. Uncommonly, patients may be febrile, with an elevated erythrocyte sedimentation rate (9). Although many cases may be related to a single traumatic event or repeated minor trauma, no history of injury is found in approximately 25% to 40% of patients (9, 10, 11). Myositis ossificans following a direct muscle injury is reported in 9% to 17% of cases (12). It is also suggested that lesions may be a reaction to infection (10). Patients are usually young adults with a mean age in the third decade (13); myositis ossificans is quite rare in children (9). Heifetz et al. estimate that only 1% of myositis ossificans occurs in the first decade (14). Approximately 80% of cases arise in the large muscles of the extremities (15), with the thigh being the most common location. Myositis ossificans is not a premalignant lesion and in most reported cases of malignant transformation, the presence of preexisting myositis ossificans is poorly documented (4); consequently, true malignant transformation is likely to be extremely rare. Local excision is generally curative. A case report documented marked clinical improvement in a mineralized lesion adjacent to the knee, following 6 months of alendronate (Fosamax) therapy, obviating the need for surgery (16). Spontaneous lesion regression and resolution are reported (17).
KEY CONCEPTS
Myositis ossificans is a benign, solitary, self-limiting, ossifying soft tissue mass.
A history of trauma is often absent and the lesion may be an incidental finding.
The most frequent symptoms are pain, tenderness, and soft tissue mass.
Patients are usually young adults with a mean age in the third decade; myositis ossificans is quite rare in children.
Approximately 80% of cases arise in the large muscles of the extremities, with the thigh being the most common location.
A distinct zoning pattern is present microscopically, in which lesional maturation progresses from an immature, central, nonossified, cellular focus to a peripheral rim of mature lamellar bone.
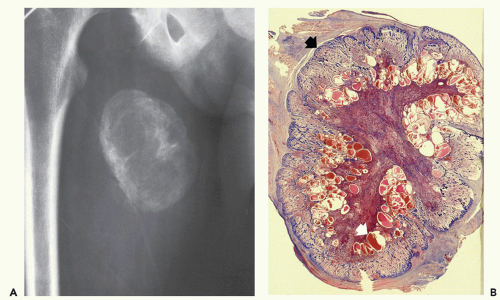
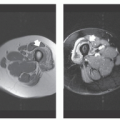
Lesions are typically well-circumscribed and rimmed by compressed fibrous connective tissue, which is frequently surrounded by or contains atrophic skeletal muscle. Typically, a distinct zoning pattern is present, in which lesional maturation progresses from an immature, central, nonossified cellular focus to osteoid, and finally to a peripheral rim of mature lamellar bone (Fig. 10.1). Central nodular fasciitis-like areas and chondro-osseous nodules may also be seen. As lesions mature, the nodular fasciitis-like areas in the intratrabecular spaces become areas of delicate fibrosis, containing thin-walled, ectatic, vascular channels that eventually become replaced by both adipose tissue and mature bone in the oldest lesions.
Imaging of Myositis Ossificans
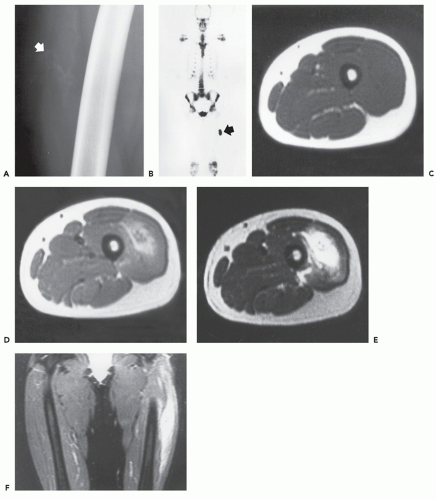
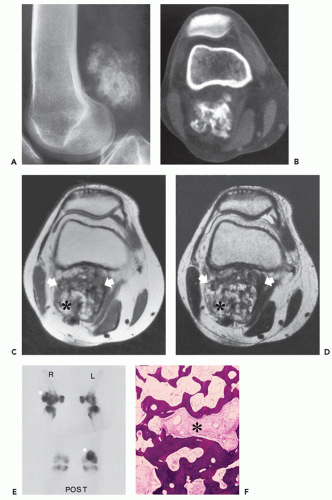
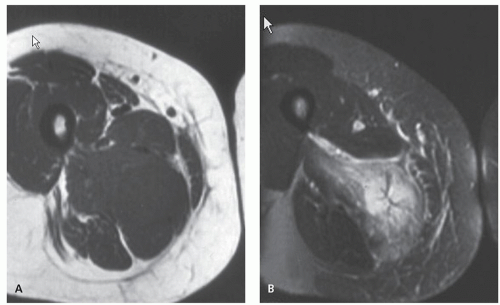
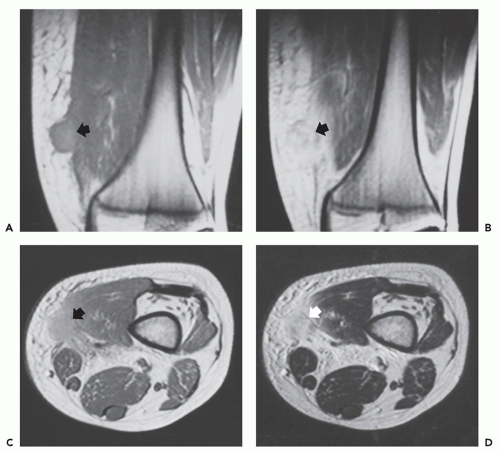
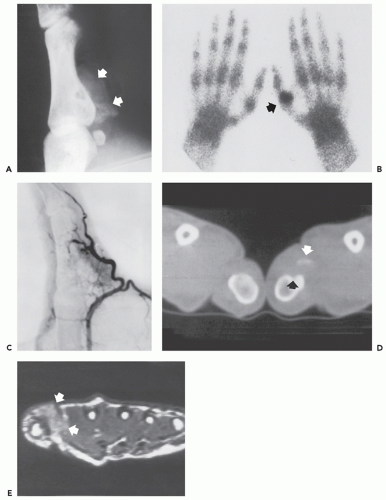
Radiographs of myositis ossificans show faint calcification within 2 to 6 weeks of onset of symptoms (5, 9). A sharply circumscribed, osseous mass is usually apparent by 6 to 8 weeks (although it may be seen much earlier), becoming smaller and mature by 5 to 6 months (11, 18, 19, 20). Lesions are often deep and may be associated with the periosteum, but they are usually separated from it by a radiolucent zone (Figs. 10.2 and 10.3) (20). Recognition of the pattern of mineralization with peripheral mature ossification is essential in establishing the radiologic diagnosis and allows differentiation from other mineralized lesions, especially extraskeletal and juxtacortical osteosarcoma (21). Short interval follow-up (3 to 4 weeks) may be invaluable in confirming the diagnosis (Fig. 10.4). It has
been our experience that mature lesions are often densely mineralized, making it more difficult to appreciate the zoning pattern of maturation (Fig. 10.5).
been our experience that mature lesions are often densely mineralized, making it more difficult to appreciate the zoning pattern of maturation (Fig. 10.5).
KEY CONCEPTS
Radiographs show:
Faint calcification within 2 to 6 weeks.
A sharply circumscribed osseous mass within 6 to 8 weeks.
Smaller, mature mass by 5 to 6 months.
Bone technetium-99m diphosphonate scintigraphy demonstrates intense focal tracer accumulation.
MR imaging features vary with lesion age:
Early lesions demonstrate a high signal intensity on T2-weighted images with associated edema.
Intermediate lesions are similar but demonstrate a rim of curvilinear decreased signal intensity corresponding to the lesions’ peripheral ossification.
Mature (late) lesions are well-defined, inhomogeneous masses with a signal intensity equal to or less than that of fat on all pulse sequences.
Active lesions demonstrate enhancement following intravenous gadolinium administration.
Bone technetium-99 m diphosphonate scintigraphy demonstrates intense focal tracer accumulation on all phases, including flow and blood-pool images (see Figs. 10.2, 10.3, 10.5, and 10.6). Early in the course, delayed static images show only mild increased tracer accumulation in the soft tissues, although this rapidly progresses to markedly increased focal tracer accumulation (22). Serial scintigraphy is used to evaluate the activity and maturity of heterotopic ossification in paraplegics (23). In such cases, when activity is reduced, the ossification is judged to be mature, and surgery may be performed with little risk of recurrence (23). Nonspecific hypermetabolic activity has been reported on fluorodeoxyglucose (FDG)-Positron emission tomography (PET) imaging (24).
Ultrasound shows an oval, hypoechoic mass with a central reflective core, which corresponds to the lesion zonal architecture seen pathologically (9). The zonal pattern may not be appreciated in early lesions and the center of the lesion will be less echogenic (9, 25). With maturity, increased hyperechogenicity is noted in the peripheral rind of mineralization and there is greater distal acoustic shadowing (9, 25).
The CT appearance of myositis ossificans is well-described and varies with the age of the lesion (18, 26, 27, 28). CT scanning of early lesions, within the first 2 weeks, shows a relatively low attenuation mass without mineralization (9). A rim of mineralization around lesions is usually well-seen after 4 to 6 weeks (see Figs. 10.4 and 10.6). The center of the mass may have decreased CT tissue attenuation (18, 26), again reflecting its similarity to nodular fasciitis (29). Mature lesions may show diffuse ossification (see Fig. 10.5). Surrounding edema may be seen on CT but is better appreciated on MR imaging (see Figs. 10.6 and 10.7) (9, 30).
In the early, active phase of myositis ossificans, arteriography shows a diffuse tumor blush and fine neovascularity (31); consequently, it may mimic a neoplasm. Mature lesions are avascular (30, 31).
The MR appearance of myositis ossificans changes with the lesion’s age, reflecting the evolving histology. Early lesions, prior to radiographically visible mineralization, demonstrate a signal intensity greater than that of fat on T2-weighted spin-echo MR images. The lesions are moderately inhomogeneous, with diffuse surrounding soft tissue edema (30, 32). On corresponding T1-weighted images, the lesion is usually isointense to skeletal muscle. Margins are poorly defined and may be recognized only secondarily by mass effect and displacement of fascial planes (see Fig. 10.2) (30, 32). Curvilinear areas of decreased signal intensity may be seen within lesions, corresponding to peripheral mineralization (see Fig. 10.4). Intermediate lesions are similar, but typically demonstrate a rim of curvilinear decreased signal intensity corresponding to the lesions’ peripheral ossification.
Irregular areas of decreased signal intensity may be seen coursing through lesions as well, also corresponding to areas of mineralization. As expected, these areas of mineralization are often best appreciated retrospectively and are far more apparent on CT.
Irregular areas of decreased signal intensity may be seen coursing through lesions as well, also corresponding to areas of mineralization. As expected, these areas of mineralization are often best appreciated retrospectively and are far more apparent on CT.
Infrequently, fluid-fluid levels may be detected, which are consistent with previous hemorrhage (33). This is not an uncommon histologic finding in the inner, most immature, portion of the lesion (see Fig. 10.4). Fluid-fluid levels are a nonspecific finding and are reported in other soft tissue lesions, including synovial sarcoma and hemangioma (33). MR signal changes compatible with edema in the adjacent bone marrow (poorly defined areas of increased signal intensity on T2-weighted
and decreased signal on T1-weighted spin-echo MR images) are also noted infrequently (6). Mature (late) lesions are well-defined heterogeneous masses showing a signal intensity approximating that of fat on all pulse sequences, with a surrounding rind of absent/decreased signal. Absent/decreased signal is also seen within the lesion, secondary to dense ossification and fibrosis (see Fig. 10.5) (30, 32). The areas of increased signal intensity seen centrally within early lesions on T2-weighted images are probably related to the extremely cellular central areas of proliferating fibroblasts and myofibroblasts within a myxoid stroma or extracellular matrix. These areas are histologically and radiologically similar in appearance to nodular fasciitis (29). Areas of hyaline cartilage may also contribute to this appearance. The inhomogeneous areas of intermediate signal, seen within late myositis ossificans on T2-weighted images, reflect areas of mature fat between bone trabeculae of the lesion. These same areas have a high signal intensity on T1-weighted images. The areas of decreased signal intensity on both pulse
sequences represent the bone trabeculae of the lesion. Areas of hemosiderin deposition from previous hemorrhage and fibrosis may also contribute to areas of decreased signal intensity on both pulse sequences. Active lesions demonstrate enhancement following intravenous contrast administration (30, 34).
and decreased signal on T1-weighted spin-echo MR images) are also noted infrequently (6). Mature (late) lesions are well-defined heterogeneous masses showing a signal intensity approximating that of fat on all pulse sequences, with a surrounding rind of absent/decreased signal. Absent/decreased signal is also seen within the lesion, secondary to dense ossification and fibrosis (see Fig. 10.5) (30, 32). The areas of increased signal intensity seen centrally within early lesions on T2-weighted images are probably related to the extremely cellular central areas of proliferating fibroblasts and myofibroblasts within a myxoid stroma or extracellular matrix. These areas are histologically and radiologically similar in appearance to nodular fasciitis (29). Areas of hyaline cartilage may also contribute to this appearance. The inhomogeneous areas of intermediate signal, seen within late myositis ossificans on T2-weighted images, reflect areas of mature fat between bone trabeculae of the lesion. These same areas have a high signal intensity on T1-weighted images. The areas of decreased signal intensity on both pulse
sequences represent the bone trabeculae of the lesion. Areas of hemosiderin deposition from previous hemorrhage and fibrosis may also contribute to areas of decreased signal intensity on both pulse sequences. Active lesions demonstrate enhancement following intravenous contrast administration (30, 34).
Although understanding of enhancement is incomplete, the vascularity seen arteriographically is likely responsible, at least in part, for the contrast enhancement seen on MR imaging (35). Surrounding enhancement reflects associated edema (26). The use of gadolinium-enhanced imaging does not facilitate diagnosis (34).
Myositis Ossificans Variants
The term myositis ossificans variant is often used for lesions with a histologic appearance similar to that of myositis ossificans, but which lack the defined zoning phenomenon with that process. These typically include panniculitis ossificans, fasciitis ossificans, florid reactive periostitis, and fibro-osseous pseudotumor of the digits. We have included soft tissue aneurysmal bone cyst in the group because of its
suggested relation with myositis ossificans in some cases; however, it is typically considered a rare distinct lesion.
suggested relation with myositis ossificans in some cases; however, it is typically considered a rare distinct lesion.
Soft Tissue Aneurysmal Bone Cyst
The soft tissue aneurysmal bone cyst is an extremely rare lesion that is histologically indistinguishable from its intraosseous counterpart (36). In a 2007 case report, Ellison et al. (37) noted the 16th reported case, including 4 in pediatric patients. The cause of soft tissue aneurysmal bone cyst remains uncertain; however, it does show overlapping morphologic features with those of myositis ossificans (36). Primary aneurysmal bone cyst will show a clonal recurrent rearrangement gene on chromosome 17p13 that encodes ubiquitin-specific protease 6 (USP6) in approximately 70% of primary lesions (38). This translocation is not identified in secondary aneurysmal bone cysts, but has been identified in myositis ossificans, suggesting that a subset of “myositis ossificans-like lesions” are the early phases in the formation of soft tissue aneurysmal bone cyst (37, 38); although currently, these lesions are considered distinct clinicopathologic entities (36, 39).
KEY CONCEPTS
Soft tissue aneurysmal bone cyst is extremely rare.
The lesion is histologically indistinguishable from its intraosseous counterpart.
It shows overlapping morphologic features with those of myositis ossificans; recent genetic studies suggest that these lesions may be related.
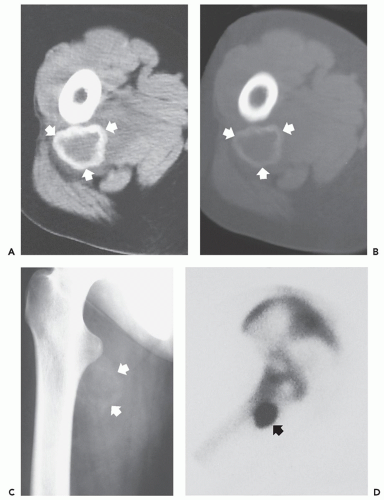
Radiographs and CT scanning demonstrate a wellorganized, peripherally mineralized mass.
MR imaging reveals a hemorrhagic lesion with multiple fluid-fluid levels and no solid component, except for thin intralesional septa.
Grossly, the lesion is composed of blood-filled cystic spaces. The walls of the hemorrhagic cysts are composed of bland, spindle-shaped fibroblasts that form a fascicular or storiform pattern with associated multinucleated osteoclasts (36). These lesions are typically well-defined and contain a layer of reactive bone, prominently rimmed by osteoblasts (36).
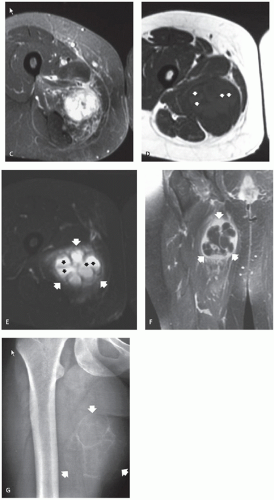
On radiographs and CT scanning, soft tissue aneurysmal bone cyst demonstrates a well-organized, peripherally mineralized mass, resembling myositis ossificans (40). MR imaging reveals a hemorrhagic lesion with multiple fluidfluid levels and no solid component, except for thin intralesional septa (36, 38, 40). Although only limited examples are available, MR imaging will show time-dependent changes from a heterogeneous mass to a fluid-filled mass with peripheral and septal enhancement (Fig. 10.8) (38).
Panniculitis Ossificans and Fasciitis Ossificans
Lesions with a histologic appearance similar to that of myositis ossificans, but which are located in the subcutis, are termed panniculitis ossificans. Panniculitis ossificans is seen most commonly in the subcutaneous tissues of the
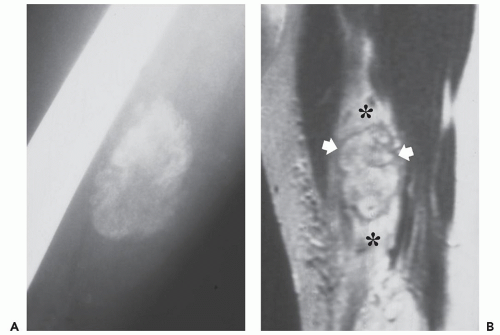
upper extremities in women (41). These lesions demonstrate a less prominent zoning phenomenon. When the lesion occurs in the fascia, it is termed fasciitis ossificans (4, 41). Although these lesions are not specifically addressed in the imaging literature, their radiologic appearance is similar to that described for myositis ossificans.
upper extremities in women (41). These lesions demonstrate a less prominent zoning phenomenon. When the lesion occurs in the fascia, it is termed fasciitis ossificans (4, 41). Although these lesions are not specifically addressed in the imaging literature, their radiologic appearance is similar to that described for myositis ossificans.
KEY CONCEPTS
Panniculitis ossificans is seen most commonly in the subcutaneous tissues of the upper extremities in women.
When the lesion occurs in the fascia, it is termed fasciitis ossificans.
Although these lesions are not specifically addressed in the imaging literature, their radiologic appearance is similar to that described for myositis ossificans.
The imaging appearances of panniculitis ossificans and fasciitis ossificans are likely similar to myositis ossificans.
The appearances of panniculitis ossificans and fasciitis ossificans are not specifically addressed in the imaging literature. Our limited experience with these lesions suggests that their imaging appearance is similar to that described for myositis ossificans (Fig. 10.9).
Florid Reactive Periostitis and Fibro-Osseous Pseudotumor of the Digits
The terms florid reactive periostitis, florid reactive periostitis of the tubular bones of the hands and feet, and fibroosseous pseudotumor of the digits are used to describe a variant of myositis ossificans that occurs predominantly in the fingers, and less commonly in the toes, of young to middle-aged adults (3, 42, 43, 44, 45). Typically arising in association with the periosteum, it (as the other myositis ossificans variants) lacks the well-defined zoning phenomenon seen in conventional myositis ossificans. Lesions are found most often in the index finger, followed by the middle and little fingers; although they may be seen in any of the digits (42). Digital lesions are found most frequently in the proximal phalanx, followed by the distal and middle phalanx (42). Patients usually present with fusiform swelling or mass. Rare lesions in other locations have been reported (43, 46, 47).
KEY CONCEPTS
Florid reactive periostitis is also termed:
Florid reactive periostitis of the tubular bones of the hands and feet.
Fibro-osseous pseudotumor of the digits.
Typically located in the hand, and less commonly, in the feet.
In the hand, lesions are most often in the index finger, followed by the middle and little fingers.
Patients are usually young, with a mean age of 30 years.
Males and females are affected equally.
Radiographs will show a mass; about half will have calcification and about half will have periosteal thickening.
The lesion generally will not demonstrate the peripherally mature pattern of ossification seen in myositis ossificans.
Local recurrence is uncommon.
Patients with florid reactive periostitis (fibro-osseous pseudotumor of the digits) are usually young, with a mean age of about 30 years. Lesions are relatively uncommon, and in a 10-year review of soft tissue tumors seen by the Department of Soft Tissue Pathology at the Armed Forces Institute of Pathology (AFIP), Washington, DC, only 12 cases of fibro-osseous pseudotumor were diagnosed. The average patient age was 32 years (range 4 to 64 years); females were affected twice as often as males (2). Other published series note that patients with fibro-osseous pseudotumor are on average more than a decade older than those with myositis ossificans; and males and females are affected equally (13).
Myositis ossificans and florid reactive periostitis represent the same pathological process; minor histological differences are believed to be related to the different site of involvement (13). When these diagnoses are suspected clinically and radiologically, the information should be shared with the pathologist. In a review of 50 cases of myositis ossificans and 14 cases of fibro-osseous pseudotumor by de Silva and Reid, a malignant diagnosis was suggested by the referring pathologist in 23% of the cases of myositis ossificans and 9% of the cases of fibro-osseous pseudotumor (13). It is also suggested that florid reactive periostitis is related to BPOP (44, 48). The nature of the relationship is discussed more fully in the section on BPOP.
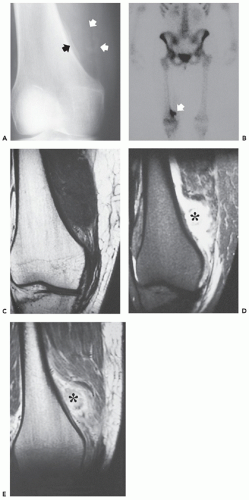
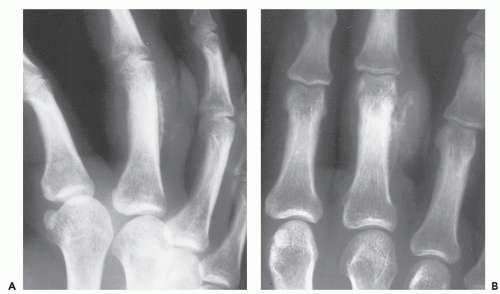
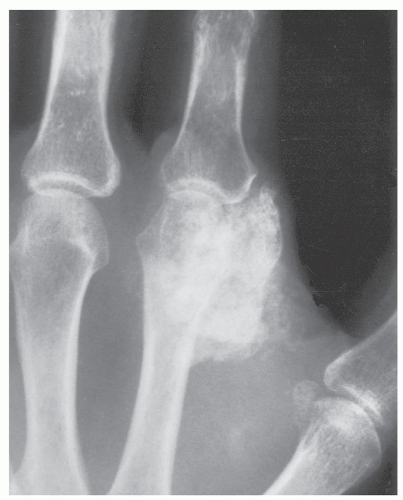
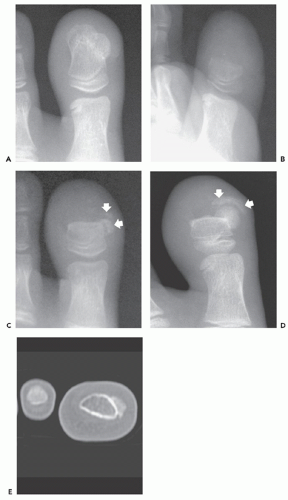
Radiographs of patients with florid reactive periostitis will demonstrate a soft tissue mass. Approximately half have visible calcification and a similar number have focal periosteal thickening (42, 49, 50). A lucent band is typically present between the density and the normal adjacent cortex (Figs. 10.10, 10.11, 10.12) (50). The lesion, especially in its early phase, may mimic a juxtacortical osteosarcoma, although such lesions are quite rare in the digits. The lesion may not demonstrate the peripherally mature pattern seen in myositis ossificans (42). Cortical erosion may
occasionally be seen (Fig. 10.13) (42). The radiographic differential also often includes periosteal chondroma and chondroma of soft parts. Noncalcified lesions may mimic a giant cell tumor of tendon sheath.
occasionally be seen (Fig. 10.13) (42). The radiographic differential also often includes periosteal chondroma and chondroma of soft parts. Noncalcified lesions may mimic a giant cell tumor of tendon sheath.
Ehara et al. (51) reported the imaging findings in two cases. CT scan in one case, with the lesion between the bases of the first and second metacarpals, showed a soft tissue mass having rim-like mineralization at the periphery, with associated periosteal new bone. MR imaging in this case showed a poorly defined, high signal intensity mass on T2-weighted images, with a thin peripheral rim of decreased signal corresponding to the calcification identified
on CT. MR imaging in a second case involving the ring finger, also showed a lobulated mass around the base of the finger, with relatively decreased signal intensity on both T1- and T2-weighted images. A thin rim of markedly decreased signal was also seen, although it was not correlated. Associated marrow edema was also noted (see Fig. 10.13).
on CT. MR imaging in a second case involving the ring finger, also showed a lobulated mass around the base of the finger, with relatively decreased signal intensity on both T1- and T2-weighted images. A thin rim of markedly decreased signal was also seen, although it was not correlated. Associated marrow edema was also noted (see Fig. 10.13).
Bizarre Parosteal Osteochondromatous Proliferation
Bizarre parosteal osteochondromatous proliferation (BPOP), sometimes termed Nora lesion, is a histologically and radiologically distinct lesion initially described by Nora in 1983, in a report of 35 cases affecting the tubular bones of the hands and feet (52, 53). Subsequent reports documented that although most cases involve the small tubular bones, long bone involvement may be seen (54, 55, 56, 57, 58, 59). The lesion arises from the surface of bone on the outer aspect of the periosteum and appears as a calcified mass broadly attached to the cortex (55). Patients are typically adults, with the mean patient age in the fourth decade, although BPOP is reported in children and older adults (53, 56, 57, 58, 59). Overall, males and females are affected equally, although this varies somewhat between reported series.
BPOP was initially described in the hands and feet, with these anatomic areas representing 55% and 15%, respectively, of all cases (53, 56). Long bone lesions are present in about 25% to 27% of cases (52, 56). Although none of the patients in the original report had a history of trauma, previous injury was associated with the lesion in a number of subsequent reports: Smith et al. reported a history of trauma in five (71%) of seven cases (53, 54, 56, 57, 59). The dominant location of the lesion, within the hands, also suggests trauma as a possible initiating event (52). BPOP is also reported to occur adjacent to posttraumatic myositis ossificans (54).
KEY CONCEPTS
Patients with bizarre parosteal osteochondromatous proliferation (BPOP) are typically adults, with the mean patient age in the fourth decade.
It occurs most frequently in the hands (55%), feet (15%), and long bones (25% to 27%).
Trauma is implicated as a cause.
Symptoms are usually related to the size of the lesion.
Excision is the usual treatment, with local recurrence in 20% to 58%.
Radiographs reveal a well-defined, pedunculated or sessile mass.
Lesions arise from the cortical surface without altering bone architecture.
A cleavage plane between the mass and the cortex may be seen.
MR imaging is nonspecific.
Patient symptoms are usually related to the size of the lesion. Excision is curative and the usual treatment. Local recurrence following primary excision is common, with local recurrence rates of 20% to 58% (48, 52, 53, 56, 59). Subsequent recurrence is reported in about a quarter of patients (53, 56). Although most cases are treated surgically, those followed with serial radiographs show slow progressive growth (57, 59).
Histologically, the lesion is a disordered mass of bone, cartilage, and fibrous tissue that has a broad-based attachment to the underlying cortex and a gross appearance similar to that of a small osteochondroma. The lesion shows hypercellularity with marked proliferative activity, irregular bony cartilaginous interfaces, and enlarged, bizarre, and binucleate chondrocytes (53, 57). The histologic appearance may mimic that of a chondrosarcoma (53). The fibrocartilagenous and myxoid tissue may be abundant in some cases (59). Early lesions usually show an absence of bone attachment, with a solid attachment developing over time (59). In spite of the high rate of recurrence and the disturbing histologic appearance of these lesions, malignant transformation and metastases are not reported.
More recently, it has been suggested that florid reactive periostitis, BPOP, and turret osteochondroma may represent distinct points in a continuing spectrum of reactive lesions that follow an initiating, likely traumatic, event (48, 52, 60, 61). Dorfman described florid reactive periostitis as the initial stage of this spectrum, composed of spindle cells with minimal osteocartilaginous proliferation, followed by BPOP, with more prominent bone and cartilage, and finally, a turret osteochondroma with
a mature bone base and cartilage cap (62). As these reactive lesions are treated surgically, definitive proof for this continuum is still needed, although isolated cases support this hypothesis (48, 52, 60). Despite the proposed relationship among these lesions, it is of interest that there are few recurrences with florid reactive periostitis, whereas recurrence may be seen in more than half of those patients with BPOP (48, 52, 53, 56, 59, 63). Chromosomal translocations have also recently been reported in BPOP suggesting a neoplastic, rather than a reactive etiology (63, 64, 65).
a mature bone base and cartilage cap (62). As these reactive lesions are treated surgically, definitive proof for this continuum is still needed, although isolated cases support this hypothesis (48, 52, 60). Despite the proposed relationship among these lesions, it is of interest that there are few recurrences with florid reactive periostitis, whereas recurrence may be seen in more than half of those patients with BPOP (48, 52, 53, 56, 59, 63). Chromosomal translocations have also recently been reported in BPOP suggesting a neoplastic, rather than a reactive etiology (63, 64, 65).
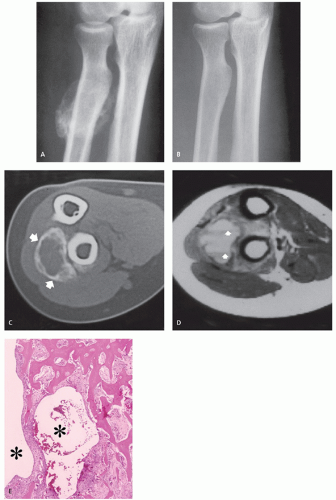
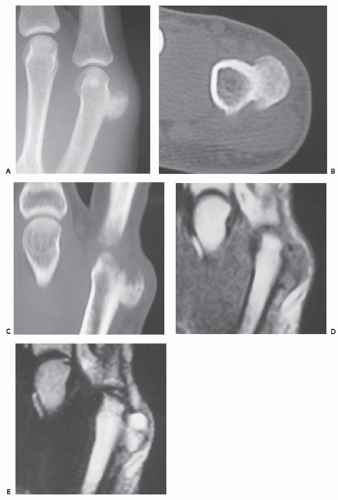
Radiographs reveal a well-defined, pedunculated, or sessile mass arising from the cortical surface of bone without alteration of the architecture of the underlying cortex (Figs. 10.14 and 10.15) (57). The osteochondromatous excrescence typically shows prominent mineralization and a broad-base attachment to the underlying cortex, and lacks the cortical and medullary continuity seen in
an osteochondroma (57, 59). The majority of lesions (67%) will present as an osseous mass (52) A cleavage plane between the mass and the cortex may be observed in less than 10% of cases (52, 59). Periosteal reaction is absent (57), but has been reported in one case (63).
an osteochondroma (57, 59). The majority of lesions (67%) will present as an osseous mass (52) A cleavage plane between the mass and the cortex may be observed in less than 10% of cases (52, 59). Periosteal reaction is absent (57), but has been reported in one case (63).
CT scan shows a normal contour to the native cortex, as well as a lack of cortical and medullary continuity (see Figs. 10.14 and 10.15). Helliwell et al. (55) described a case in the radius with focal cortical invasion. MR imaging is nonspecific, demonstrating an intermediate to high signal intensity on fluid-sensitive sequences, with mildly heterogeneous to marked contrast enhancement (52, 55). Recurrent lesions show similar radiologic features (see Fig. 10.15).
Fibrodysplasia Ossificans Progressiva
Fibrodysplasia ossificans progressiva (FOP), is a rare, slowly progressive disorder characterized by fibroblastic proliferation, subsequent calcification, and ossification of subcutaneous fat, tendons, aponeuroses, ligaments, and skeletal muscle. It is usually associated with symmetric malformation of the digits, especially the great toes and thumbs; short, broad femoral necks; and vertebral anomalies. The bone-forming lesions in FOP are typically precipitated by local trauma, underscoring the previous designation of myositis ossificans progressiva; however, this designation is a misnomer and is no longer used. FOP is a disease that primarily affects connective tissue and changes in muscle are secondary (66).
The disease is usually sporadic but may be inherited as an autosomal dominant trait with variable penetrance (67, 68). FOP is quite rare, with an estimated prevalence of 0.6 per million persons (69). Patients are usually children, with about half presenting by 2 years of age (66, 69), usually with localized soft tissue swelling, which may be accompanied by pain, edema, and a low-grade fever (66). These early lesions are typically in the neck or paraspinal region (70). Appendicular and distal lesions usually appear later (71). Previous trauma is inconsistently identified as a cause of the soft tissue lesions (69). In time, the soft tissue swelling resolves and the soft tissue masses coalesce, fibrose, and calcify, leading to the formation of “bony bridges” that cause restriction of respiration and ambulation and skeletal contractures. The ossification process may occur quickly, with doughy nodules progressing to well-defined ossified lesions within weeks (72), although ossification is usually not identified until 4 to 6 months following the appearance of a mass (73). The average age of onset of ossification is 5 years (73); most affected individuals have soft tissue heterotopic bone formation by 10 years of age (69, 71). By 15 years of age, 95% of patients have severely restricted upper limb mobility, and patients are usually wheelchair-bound by the third decade (74).
KEY CONCEPTS
Fibrodysplasia ossificans progressiva (FOP) is a rare, progressive disorder characterized by fibroblastic proliferation, calcification, and ossification of subcutaneous fat, tendons, aponeuroses, ligaments, and skeletal muscle.
It is usually sporadic and may be an autosomal dominant trait with variable penetrance.
The average age of onset of ossification is 5 years; severely restricted upper limb mobility is seen by 15 years of age.
Heart, diaphragm, larynx, tongue, and sphincter muscles are spared, as are all smooth muscle structures.
Diagnosis is based on characteristic skeletal deformities and soft tissue ossifications.
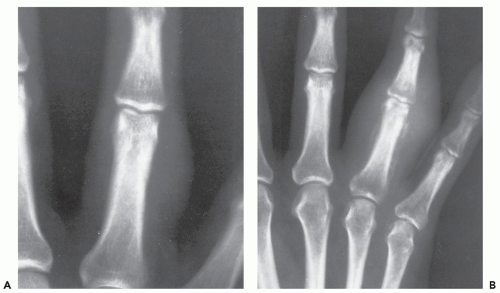
Symmetric hypoplasia of the great toes is the most characteristic skeletal deformity.
Similar abnormalities may be seen in the thumbs.
Less common deformities include vertebral hypoplasia and short, broad femoral necks.
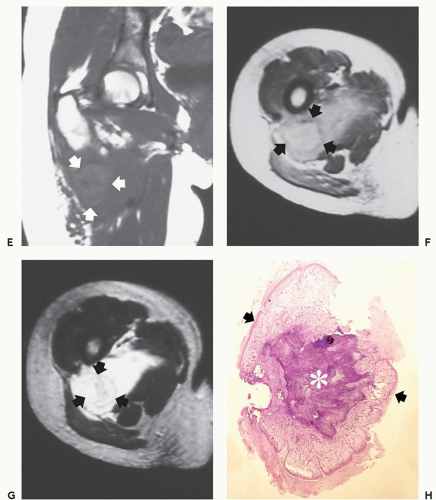
Although the ectopic ossification is independent of skeletal maturation, maturing ectopic ossification forms rigid synostoses between the normal skeletal structures (71). The rigid synostoses bridge and immobilize joints, markedly restricting motion (Figs. 10.16 and 10.17) (71). The heart, diaphragm, larynx, tongue, and sphincter muscles are spared, as are all smooth muscle structures (74). The diagnosis is usually made on the basis of the characteristic skeletal deformities and soft tissue ossifications (70). The most characteristic skeletal malformation is seen in the great toe, with shortening of the first metatarsal and proximal phalanx. This anomaly is present at birth and is usually associated with a monophalangic first toe (fused phalanges) (71). Similar abnormalities may be seen in the thumbs. Hand abnormalities are never present as an isolated finding (71). Less common skeletal deformities include vertebral hypoplasia, variable degrees of vertebral fusion, and short, broad femoral necks (71). Ossification of ligamentous insertions may produce exostosis-like abnormalities. Kyphoscoliosis is a common finding and is the result of asymmetric, heterotopic ossification involving the rib cage and paraspinal regions (71). The temporomandibular joint is involved in almost three quarters of patients and is often the last joint affected (75). Delayed diagnosis is not uncommon, even after the onset of ectopic ossification (70); Connor and Evans (73) report an average delay of greater than 3 years in such patients. The disease course is characterized by remissions and exacerbations. Local trauma, including surgery and intramuscular injections, are implicated in disease progression (66, 72, 76, 77); however, most often the cause of disease exacerbations is unknown (78). Influenza-like viral illnesses are linked to disease flareups, which may be the result of previously unrecognized viral muscle injury (76). Some patients succumb to the disease at an early age. Long-term survival is not uncommon; however, most patients do not have a normal life span (69). Various treatments are used for FOP, including steroids, mineral-binding agents, and calcium-blocking
agents; although no effective long-term treatment has been found (67, 69, 79, 80). Newer evidence suggests that 13-cis-retinoic acid (Accutane) may reduce the rate of involvement of previously unaffected joints (78).
agents; although no effective long-term treatment has been found (67, 69, 79, 80). Newer evidence suggests that 13-cis-retinoic acid (Accutane) may reduce the rate of involvement of previously unaffected joints (78).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree