Abstract
Objective
The objective of this study was to evaluate the clinical feasibility of deep learning reconstruction-accelerated thin-slice single-breath-hold half-Fourier single-shot turbo spin echo imaging (HASTE DL ) for detecting pancreatic lesions, in comparison with two conventional T2-weighted imaging sequences: compressed-sensing HASTE (HASTE CS ) and BLADE.
Methods
From March 2022 to January 2023, a total of 63 patients with suspected pancreatic-related disease underwent the HASTE DL , HASTE CS , and BLADE sequences were enrolled in this retrospectively study. The acquisition time, the pancreatic lesion conspicuity (LC P ), respiratory motion artifact (RMA), main pancreatic duct conspicuity (MPDC), overall image quality (OIQ), signal-to-noise ratio (SNR), and contrast-noise-ratio (CNR) of the pancreatic lesions were compared among the three sequences by two readers.
Results
The acquisition time of both HASTE DL and HASTE CS was 16 s, which was significantly shorter than that of 102 s for BLADE. In terms of qualitative parameters, Reader 1 and Reader 2 assigned significantly higher scores to the LC P , RMA, MPDC, and OIQ for HASTE DL compared to HASTE CS and BLADE sequences; As for the quantitative parameters, the SNR values of the pancreatic head, body, tail, and lesions, the CNR of the pancreatic lesion measured by the two readers were also significantly higher for HASTE DL than for HASTE CS and BLADE sequences.
Conclusions
Compared to conventional T2WI sequences (HASTE CS and BLADE), deep-learning reconstructed HASTE enables thin slice and single-breath-hold acquisition with clinical acceptable image quality for detection of pancreatic lesions.
1
Introduction
Magnetic resonance imaging (MRI), with its excellent spatial resolution and absence of radiation, plays a vital role in the diagnosis of pancreatic diseases . T2-weighted imaging (T2WI), including fat-suppressed T2WI, as an essential component of pancreatic MRI sequences, enabling the detection and diagnosis of pancreatic structures and lesions . However, conventional T2-weighted turbo spin echo (TSE) sequences are time-consuming (several minutes) and prone to motion artifacts due to multiple breath-hold and respiratory-triggered acquisitions . These motion or respiratory artifacts can undermine radiologists’ confidence and accuracy in pancreatic diagnosis . Furthermore, the pancreas, being thinner and surrounded by adjacent tissues, requires higher image quality compared to other abdominal organs[ , ]. Image fuzziness, misregistration, reduced signal-to-noise ratio, or contrast can obscure significant pancreatic lesions and anatomical structures.
Despite the development of additional techniques such as respiratory-triggering or k-space filling techniques, which have somewhat reduced respiratory motion artifacts, they still require long image acquisition time and multiple breath-holds[ , ]. Single-shot T2WI techniques, such as single-shot fast spin echo (single-shot FSE) and the half-Fourier acquisition single-shot turbo spin echo (HASTE), have been considered as alternatives for reduce motion artifacts due to their faster acquisition times and improved motion stability at baseline[ , ]. However, compared to conventional T2W TSE, the imaging contrast and quality in single-shot FSE T2WI techniques exhibit reduced imaging contrast and quality due to longer echo training . In contrast, HASTE T2WI acquired less k-space data, allowing for shorter scan times and further reduction of motion artifacts at the cost of reduced spatial resolution and lesion conspicuity.
More acceleration methods have been developed and applied in the clinic to obtain T2W images with shorter acquisition time and higher quality [ , ]. For example, compression sensing (CS), an acceleration technique, has been employed to achieve faster scanning with improved image quality by bypassing the Nyquist-Shannon sampling criterion and utilizing non-coherent k-space sampling . However, despite advancements in sparse transform algorithms, the reconstruction of undamaged and clear images from randomly and highly under-sampled k-space data, and the preservation of microscopic anatomical structures, remains challenging when utilizing the CS acceleration technique. Additionally, the incorporation of trainable deep learning (DL) algorithms, such as convolutional neural networks (CNNs), into reconstruction techniques to improve the inherent defects of conventional imaging scans has been intensively developed [ , ]. For instance, in our center, several modified T2WI based on DL-based reconstruction, such as single-breath-hold T2WI and artificial intelligence-assisted compressed sensing single-shot fluid-attenuated inversion recovery, have successfully applied in the liver and brain lesion detection[ , ]. DL reconstruction-accelerated HASTE (HASTE DL ) has shown its advantages in abdominal MRI[ , ]. However, there is a lack of studies on DL techniques specifically applied to pancreas T2WI.
Therefore, the objective of our study was to evaluate the clinical feasibility of thin-slice single-breath-hold HASTE DL for the detection of pancreatic lesions and compare its performance with HASTE based on compressed sensing (HASTE CS ) and BLADE techniques.
2
Materials and methods
This retrospective study was approved by the Institutional Review Board of our hospital (approval no. B2020–346R), and the informed consent was waived. The process in our study strictly adheres to ethical- and hospital-related guidelines and regulations.
2.1
Participants
From March 2022 to April 2023, 63 patients with the suspected pancreatic-related disease who underwent abdominal 3T MRI were enrolled.
The inclusion criteria of this study were as follows: a) patients with radiologically assessable pancreatic lesions, with or without surgical or endoscopic puncture pathology confirmation; b) all patients with complete clinical and radiological data, including images from at least three sequences of HASTE DL and HASTE CS , and BLADE. Additionally, the exclusion criteria consisted of: a) patients with claustrophobia or unable to complete the examination, b) poor image quality due to severe metallic or motion artifacts.
2.2
Image acquisition
All patients in the study underwent MRI using a 3-T MR scanner (MAGNETOM Prisma; Siemens Healthcare, Erlangen, Germany). The examination protocol consisted of conventional T1WI, diffusion-weighted imaging (DWI, b = 0, 800 s/mm 2 ), BLADE, and the research applications HASTE DL and HASTE CS , which were randomly conducted before contrast. Then, dynamic enhanced imaging was conducted using gadolinium diethylenetriamine pentaacetic acid (Gd-DTPA, Bayer HealthCare, dose, 0.1 mmol/kg; rate, 2 mL/s). The arterial, portal, and delayed phases were obtained at 20, 90, and 180 s, respectively. In this study, the total scan time was 102 s for BLADE with four breath-holds and 16 s for both HASTE DL and HASTE CS with single breath-hold. The rest of the detailed protocols of HASTE DL , HASTE CS , and BLADE are listed in Table 1 .
| HASTE DL | HASTE CS | BLADE | |
|---|---|---|---|
| TR (ms) | 628 | 628 | 3000 |
| TE (ms) | 105 | 105 | 114 |
| Matrix | 320×320 | 320×320 | 320×320 |
| Field of view (mm 2 ) | 360×360 | 360×360 | 360×360 |
| Voxel size (mm 3 ) | 1.1 × 1.1 × 4 | 1.1 × 1.1 × 4 | 1.1 × 1.1 × 4 |
| Section thickness (mm) | 4 | 4 | 4 |
| Slice gap (mm) | 0.4 | 0.4 | 0.4 |
| Number of sections | 26 | 26 | 26 |
| Flip angle ( ◦ ) | 130 | 130 | 120 |
| Bandwidth (Hz) | 710 | 710 | 600 |
| Acceleration | 3.3 | 3.3 | 2 |
| Respiratory control | single breath-hold | single breath-hold | four breath-holds |
| Single breath-hold time (s) | 16 | 16 | 18 |
| Fat suppression | SPAIR | SPAIR | SPAIR |
| TA (s) | 16 | 16 | 102 |
2.3
MRI image analyses
All T2WI images acquired with HASTE DL , HASTE CS , and BLADE were independently analyzed by two radiologists (Reader 1, X.X.X, and Reader 2, X.X.X, with 8 and 23 years of radiological experiences in pancreatic diseases, respectively) who were blinded to clinical, pathological, and radiological results during the review processing.
2.3.1
Qualitative imaging analysis
The pancreatic lesion conspicuity (LC P ), respiratory motion artifact (RMA), main pancreatic duct conspicuity (MPDC), and overall image quality (OIQ) were independently and subjective scored by three readers for three T2WI sequences. These indicators were evaluated according to a five-point Likert scale as follows: 1 (severe): the presence of blurry edges of pancreatic lesions or structures, heavy image artifacts, and poor image continuity that do not meet the diagnostic requirements; 2 (moderate): the presence of relatively blurry edges of pancreatic lesions or structures, heavy image artifacts, and poor image continuity that make it difficult to meet the diagnostic requirements; 3 (mild): the presence of slightly blurry edges of pancreatic lesions or structure, and acceptable image artifacts and continuity that meet the basic diagnostic requirements. 4 (fine): the presence of relatively sharp and clear edges of pancreatic lesions or structure, and negligible image artifacts and continuity that meet the diagnostic requirements; 5 (excellent): pancreatic lesions or structures with completely sharp and clear edges, no image artifacts, good continuity, and fully compliant with diagnostic requirements. Moreover, slice continuity, defined as inconsistent horizontal position between layers, was divided into discontinuity or no discontinuity. The above two radiologists analyzed the slice continuity independently. When inconsistencies arose, a third radiologist with 25 years of radiological experience was asked to evaluate and use the unified opinion for follow-up analysis.
2.3.2
Quantitative image analysis
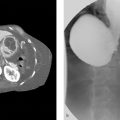
The signal intensities (SI) of the head (H), body (B), tail (T), and lesion (L) of the pancreas and the SI of the erector spinal muscle (ESM) were measured on operator-defined regions-of-interest (ROI) on the three T2WI images, respectively ( Fig. 1 ). The sizes of ROI in the vertical spine muscle and pancreatic tissue and lesions are about 50∼100 mm 2 , and the corresponding ROI is placed in the signal uniformity area, avoiding the influence of blood vessels and bones. The image signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) are calculated according to the following formulas:
SNR of pancreatic head: SNR H =SI H / SI ESM ;
SNR of pancreatic body: SNR B =SI B / SI ESM ;
SNR of pancreatic tail: SNR T =SI T / SI ESM ;
SNR of pancreatic lesions: SNR L =SI L / SI ESM ;
CNR of pancreatic lesions: CNR L =|SI L -SI ESM | / SI ESM

2.4
Statistical analysis
Statistical analyses were performed using SPSS version 26 (IBM Corp., Armonk, NY, USA). The image quality scores obtained for three T2WI sequences were compared using a paired Wilcoxon signed-rank test. P < 0.05 was considered as statistically significant and Bonferroni correction was conducted to correct for cumulative α error . Interobserver agreement was assessed using the Cohen κ coefficient . The κ values were used to interpret according to the following criteria: poor (0–0.20), fair (0.21–0.40), moderate (0.41–0.60), good (0.61–0.80), almost perfect (0.81–1).
3
Results
3.1
Patient characteristics
A total of 63 patients (mean age, 61.13 ± 11.25 years; range, 30–83 years, 34 men and 29 women;) with pancreatic lesions were enrolled in the present study for subsequent analysis.
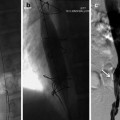
Regarding pancreatic lesions, surgical tumor resection with pathological confirmation was performed on 31 patients. Among them, 23 patients were diagnosed with pancreatic ductal adenocarcinoma (PDAC), while 8 patients had pancreatic neuroendocrine tumors (G1, n = 2 and G2, n = 6). Additionally, 11 patients received pathologically confirmed through endoscopic puncture and were diagnosed PDAC. The remaining patients were diagnosed based on the indications of abdominal MRI and clinical signs and symptoms, including pancreatic solid tumor ( n = 7), pancreatic cystic lesions ( n = 12) and suspected pancreatic inflammatory disease ( n = 2). The size of the lesions (maximum diameter) ranged from 1.1 to 7.1 cm, with a mean of (2.9 ± 1.4) cm. For the location of pancreatic lesions, there were 36 in the head of pancreas, 12 in the body of pancreas, and 19 in the tail of pancreas. Moreover, 11 cases (17.5 %) in HASTE DL showed slice discontinuities, significantly less than 18 (28.6 %) and 20 (31.7 %) cases in HASTE CS and BLADE.
3.2
Qualitative imaging analysis
The three T2WI protocols were successfully examined and analyzed in all patients. The acquisition time (AT) for both HASTE DL and HASTE CS was 16 s, while the AT for BLADE was 102 s.
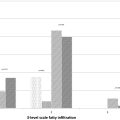
The five-point Likert scale was used to assess four qualitative image qualities (LC P , RMA, MPDC and OIQ) for HASTE DL , HASTE CS , and BLADE sequences ( Tables 2 and 4 ). In terms of pancreatic lesion analysis, a total of 65 pancreatic lesions were detected and evaluated. The LC P , RMA, and OIQ scores of HASTE DL in Reader 1 and Reader 2 were all significantly higher than those for HASTE CS and BLADE sequences (P value ranging from <0.001 to 0.003) ( Figs. 2 and 3 ). However, there were no statistical differences in these scores between HASTE CS and BLADE. For pancreatic ducts, HASTE DL achieved highest MPDC scores of 4.67±0.48 and 4.68±0.47 in reader 1 and reader 2, respectively, which were significantly higher than those of HASTE CS and BLADE (P value ranging <0.001 to 0.006) ( Fig. 4 ). In terms of MPDC, the scores for HASTE CS in both Reader 1 and Reader 2 were 4.48±0.59 and 4.49±0.56, respectively, which were higher than those of BLADE.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree