Developments in robotic interventions have greatly affected the field of interventional radiology (IR), particularly when combined with imaging modalities such as fluoroscopy and cone-beam computed tomography (CBCT). The aim of this review is to compare and evaluate the safety, precision, and clinical outcomes of fluoroscopy and CBCT-guided robotic interventions in IR. An extensive search of the literature on PubMed and Google Scholar databases was conducted up to November 2024. Searched terms included “robotic interventions,” “fluoroscopy guidance,” “cone-beam CT guidance,” and “robotic surgery.” Literature review showed improved patient outcomes in robotic-assisted procedures, with fewer complications and higher success rates especially in anatomically challenging cases. Fluoroscopy-guided robotic interventions provide real-time imaging, allowing for accurate interventions while CBCT-guided procedures offer enhanced 3D visualization, reducing radiation exposure while maintaining high diagnostic accuracy and shorter needle puncture times. Both fluoroscopy and CBCT-guided robotic interventions play a critical role in advancing interventional radiology and are expected to improve procedural outcomes in IR.
Introduction
Although slow, advancements in robotic interventions have become more prominent, enhancing precision and control in these minimally invasive procedures compared to traditional surgical techniques. Robotic interventions can also be used across a wide variety of medical specialties, such as oncology and urology. , They hold promising results as they can improve clinical outcomes and minimize human errors in a wide variety of clinical settings. Robotic interventions can also integrate advanced imaging modalities, further assisting and providing better accuracy in complex surgeries. As a matter of fact, the use of imaging to guide these minimally invasive procedures has become increasingly common in clinical practices. , ,
Various imaging modalities can be used to guide robotic interventions such as computed tomography (CT), magnetic resonance imaging (MRI), ultrasound (US), fluoroscopy. Among these, fluoroscopy and CT guidance have gained great popularity. Fluoroscopy guided interventions offer real-time imaging, therefore enhancing control during procedures, while CT guided procedures offer prevention from unnecessary radiation by decreasing procedure time while maintaining good precision. ,
The focus of this review is to discuss fluoroscopy and cone beam CT guided robotic interventions by assessing key factors such as safety, precision, and clinical outcome. Ultimately, this review highlights the potential of these techniques to improve patient outcomes, reduce radiation exposure and establish promising results.
Methods
An extensive literature review was conducted using PubMed and Google Scholar databases as of November 2024. We searched several terms including “robotic interventions,” “image-guided interventions,” “fluoroscopy-guided procedures,” “cone-beam CT-guided interventions,” “robotic-assisted biopsy,” and “minimally invasive robotic surgery.” Important references from identified studies were also reviewed. The search was limited to English language articles, focusing on studies that discussed the use of robotic systems in interventional radiology and their impact on clinical outcomes, safety, and procedural efficiency.
Image-guided robotic interventions in interventional radiology
Interventional radiology (IR) is a relatively new field that relies on radiological image-guidance to perform minimally invasive procedures with high precision. When compared to traditional invasive surgeries, IR procedures proved to be safe with less complications, better cost-effectiveness, and shorter recovery periods and hospital stay.
In the year 2000, the introduction of the first Food and Drug Administration (FDA) robotic system, the Vinci system, greatly influenced the field of IR. This system, now greatly used in general laparoscopic surgeries and minimally invasive prostatectomies, showed increased precision and accuracy during tissue manipulation. Therefore, these advancements sparked interest in the use of image-guided robotic interventions.
Robotic systems in IR can be used for percutaneous interventions or endovascular procedures. A significant advantage of robotics in IR is the potential to decrease radiation exposure for the operator through the use of a remote console and to decrease procedure times and therefore radiation exposure for the patient as well. In fact, a study showed that the use of the Magellan robotic system for transarterial chemoembolization (TACE) showed an 80% reduction in radiation exposure for the operator, highlighting the significant safety benefit for healthcare providers who would otherwise be exposed to high radiation during IR procedures.
Advancements in robotic procedures include the use of image-guided needle insertion systems that play an important role in oncology, where precision is essential. , , In fact, a pilot study assessing the feasibility and safety of the EPIONE robotic system for percutaneous liver tumor ablation, showed promising results including an 83.3% 6 months tumor control rate, minimal needle adjustments and side effects. Added to that, a study comparing intraoperative CT, cone-beam CT (CBCT), and robotic CBCT (rCBCT) for spinal pedicle screw instrumentation found that CBCT and rCBCT offered independent handling and around-the-clock availability with high screw placement accuracy of more than 92%.
Fluoroscopy-guided robotic interventions
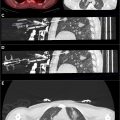
The use of fluoroscopy in IR procedures is very important as it allows continuous real-time imaging providing high accuracy. However, high radiation exposure to the physician is a main disadvantage. Robotic systems decrease this exposure by enabling remote operation by the physician. In fact, a study published by Solomon et al. showed that robotics can completely eliminate physician’s exposure to radiation. Another study showed that the use of fluoroscopy-guided robotic systems can help physicians perform biopsies in anatomically challenging locations, with minimal high grade side effects and with good precision.
Fluoroscopy guided robotic interventions are also widely used in Urology. In fact, a study comparing robot-assisted fluoroscopy-guided (RAF) endoscopic combined intrarenal surgery (ECIRS) with US-guided ECIRS, RAF-ECIRS group achieved successful renal access with a median of 1 needle insertion attempt, 3.6 minutes for needle insertion time, 10 seconds for needle alignment, and 1.2 minutes of irradiation time. In comparison, the USG group had similar success but required 8.1–17.8 minutes of fluoroscopy time, whereas the RAF group ranged from 8.9 to 18.8 minutes, indicating a comparable time frame between both methods. Another study comparing robot-assisted fluoroscopy-guided renal access with US-guided renal access for nephrolithomy found that fluoroscopy guidance had a 100.0% success rate of puncture compared to 70.6% in US guidance. Moreover, self-reports from Urologist also showed more satisfaction with fluoroscopy. ,
Cone-beam CT-guided robotic interventions
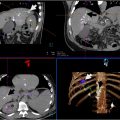
The study investigates radiation exposure during mobile cone-beam CT (CBCT)-guided robotic-assisted bronchoscopy (RAB), focusing on both patients and staff. It analyzes 198 procedures, identifying key factors contributing to increased radiation doses. The research found that both patient and staff exposure levels remained within safety thresholds. Higher patient radiation was associated with obesity, smaller lesions, and inadequate sampling. For staff, particularly the bronchoscopist, radiation exposure correlated with the number of CBCT spins and targeted lesions. The study highlights the importance of optimizing procedures and protective measures to minimize radiation risks while enhancing diagnostic accuracy.
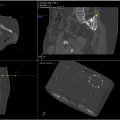
A study published in 2023 by Key et al. discussed the applications of cone-beam CT (CBCT) in interventional radiology, with focus on in needle guidance and augmented fluoroscopy overlay. The study proved the effectiveness of CBCT in procedures several procedures including epidural steroid injections, celiac plexus blocks, pudendal nerve blocks, and more complex interventions like spine ablations. The study also mentioned that despite these advantages, CBCT remains underutilized, partly due to unfamiliarity with the technology among practitioners.
Some studies focus on the use of 3-dimensional C-arm C CBCT and found that it improves precision in complex vascular and nonvascular interventions, such as hepatic arterial procedures, portal vein embolization, and spinal interventions, by allowing real-time imaging and planning. The study also showed that CBCT reduces radiation exposure, although 1 limiting factory would be the long setup and reconstruction times. Another study investigating the use of C-arm cone-CBCT virtual-navigation guidance for percutaneous transthoracic needle biopsy (PCNB) of small lung nodules found similar results. It involved 105 patients with 107 small lung nodules. The study demonstrated that CBCT virtual-navigation-guided PCNB is highly accurate (98%), sensitive (96.7%) and specific of (100%). Complications were minor and included a case of pneumothorax and hemoptysis and radiation exposure was low(5.72 mSv). A third study evaluating the diagnostic accuracy and complications of CBCT-guided percutaneous transthoracic needle biopsy (PTNB) for small lung nodules showed very similar results. The results were as follow: diagnostic accuracy (98.2%), sensitivity (96.8%) and specificity (100%), mean effective radiation dose (8.6 mSv).
One study investigating the radiation dose associated with the use of CBCT combined with shape sensing robotic-assisted bronchoscopy (ssRAB) for the biopsy of pulmonary lesions found that the procedure had a relatively low radiation dose compared to other image-guided biopsy techniques (mean cumulative radiation dose of 63.5 mGy for air kerma (CAK) and 22.6 Gy·cm² for dose area product (DAP). The study also noted a low complication rate.
In urology, CBCT has also been widely used. A study evaluated the feasibility of using real-time ultrasound (US) and cone-beam CT (CBCT) fusion imaging to guide percutaneous ablation of small renal tumors (<2 cm). In a sample of 5 patients with 6 renal lesions, the US/CBCT fusion technique showed to be accurate and achieved a 100% technical success with no recurrence at follow-up in all the cases. No major complications were reported in this study.
Comparative analysis: Fluoroscopy vs. CBCT-guided techniques
We will start by discussing the results of some papers focusing on fluoroscopically and CBCT-guided procedures. The first study investigated 89,549 fluoroscopically-guided interventions, identifying those with radiation doses >5000mGy as having “significant” exposure as defined by the Society of Interventional Radiology. The study reported that while such high-dose procedures are rare, they are more commonly performed by non-radiologists who may not have the same training in radiation safety therefore the need for strict safety protocols.
Another study compared the diagnostic performance and safety of fluoroscopy CT (FCT) and cone-beam CT (CBCT) guidance in percutaneous transthoracic needle biopsy (PTNB) of lung base nodules. The study shows that both techniques had comparable technical success rates (around 98%), particularly for lesions that are more than 2 cm in diameter. However, the study showed that for smaller lesions, CBCT offered advantages by reducing radiation exposure and complications like pneumothorax. Thus, this study concluded that CBCT is preferable for challenging, smaller nodules, while both techniques are effective for larger ones. These results were also supported by a study examining the use of CBCT guidance for percutaneous lung biopsies (PLBs) in cases where bronchoscopy fails to provide a histopathologic diagnosis. The study showed high diagnostic accuracy (91.7%), with a sensitivity of 90%, and a specificity of 100. The study also noted a small number of complications, including minor pneumothoraxes and 1 major complication. The CBCT guidance was found to be an effective and safe method for performing PLBs, with comparable outcomes to other imaging techniques like CT and CT fluoroscopy. A third study investigating the use of CBCT-guided bronchoscopy to improve the diagnosis of peripheral lung nodules found that CBCT guidance could enhance both special and diagnostic yields. While the pre-CBCT diagnostic yield was 50%, the post-CBCT yield increased to 70% with an acceptable radiation dose.
One study comparing CBCT and CT fluoroscopy (fluoro-CT) in guiding transthoracic needle biopsy (TNB) for diagnosing lung nodules showed that both techniques demonstrated high diagnostic accuracy (96% with CBCT and 94% with fluoro-CT), high sensitivity(95% with CBCT and 92% with fluoro-CT) and a specificity of 100% for both methods. The average radiation dose was slightly lower with CBCT (median 11.1 mSv) compared to fluoro-CT (14.5 mSv), though this difference was not statistically significant. Both techniques had comparable complication rates, with pneumothorax occurring in approximately 29% of cases. the study concluded that when comparing the 2 modalities, CBCT offers the advantage of 3D imaging and better needle path visualization enhancing precision, while fluoro-CT that is more traditional also offers high effectiveness and remains widely used due to its simplicity and established protocols. Although CBCT may reduce radiation exposure and improve diagnostic yield, especially for smaller lesions, both modalities can be effectively used depending on resource availability and the operator’s familiarity with the technology ( Table 1 ).