Introduction
Radiation therapy, whether used as definitive treatment or as an adjuvant to surgery, is an essential component of treatment for many patients with head and neck cancer (HNC). Both photon (x-ray) therapy and proton therapy are standard forms of radiation therapy, and both have evolved over time with the advent of technological advances. Proton therapy has evolved from two-dimensional (2D) and three-dimensional (3D) passive scatter to the current state of the art, which is active (spot) scanning intensity-modulated proton therapy (IMPT). With ongoing improvements in terms of the sophistication of both planning and delivery, IMPT is considered likely to advance further in terms of dose distributions in the near future.
One of the main benefits of photon (x-ray) intensity-modulated radiation therapy (IMRT) relative to traditional 2D or 3D radiation therapy techniques for HNC is the ability to conform the therapeutic dose distributions around targets. This ability allows the parotid glands (as one example) to be spared relative to the dose given to the tumor, which translates to reduced xerostomia and improved quality of life compared with the traditional 2D or 3D approaches. However, despite the demonstrated clinical advantages and rapid and widespread adoption of IMRT, the all-too-common problematic acute toxic effects (e.g., mucositis, pain, odynophagia, dysphagia, fatigue, nausea/vomiting, and dysgeusia/ageusia) and late treatment-related toxic effects (e.g., xerostomia, fatigue, dysphagia, and osteonecrosis), with their associated symptoms and negative effects on important functions (e.g., swallowing, cognition, vision, voice, speech, and general activity) and overall quality of life, still remain significant problems for many patients being treated for HNC.
Moreover, clinicians are increasingly recognizing the dosimetric trade-offs and negative clinical consequences of applying the multiple oblique beams (e.g., 7–9 static fields or even 360-degree volumetric arcs) required to generate contemporary parotid-sparing IMRT plans. IMRT techniques inherently increase the irradiated volumes, and their beams traverse numerous nontarget normal tissues that are outside the treatment portals and essentially unirradiated in the pre-IMRT era. Numerous studies have shown that the dose delivered to nontarget structures by IMRT has negative clinical consequences (“beam-path toxicity”), especially for structures that are posterior or anterior to the targets. For example, even intermediate- or low-dose radiation to the brainstem to levels generally considered to be safe correlate with increased nausea and vomiting and fatigue. , Also, radiation doses to the (nontarget) anterior mandible correlate with anterior oral cavity mucositis and with dysgeusia, ageusia, minor salivary gland function, and long-term perceptions of oral moisture and oral comfort. Other investigators have also highlighted the potential negative effects of technique-driven photon (x-ray) IMRT on unnecessary dose to the skin, larynx, and esophagus and the associated toxic effects. ,
Numerous dosimetric comparison studies have demonstrated that proton therapy—active (spot) scanning IMPT in particular—can achieve better dose distributions than photon (x-ray) IMRT for numerous HN tumor subsites. In general, IMPT has been shown to achieve conformality of high-dose regions around targets that is comparable with that from IMRT but with the advantage of further reducing or even eliminating the unnecessary low-to-intermediate-range integral dose to numerous adjacent critical organs and normal tissues. The unique inherent physical properties of protons, with their lower entrance dose and zero exit dose, plus the advantage of a sharper lateral penumbra (particularly with aperture use), translate into fewer beams being required for proton therapy than for IMRT and reduction in beam competition effects relative to that for IMRT plan optimization. Thus, clinicians have sought to exploit the highly conformal and more compact dose distributions from proton therapy for treating HNCs, with the goals of further improving patient outcomes through the safer delivery of therapeutic doses to targets while minimizing or even eliminating the beam path toxicity associated with IMRT.
Passive scatter proton therapy was the first iteration of proton therapy to be used for HNC; over the past several years, more treatment centers are coming online with more advanced active (spot) scanning and IMPT capabilities, and the implementation of proton therapy for HNC has expanded. At the University of Texas MD Anderson Cancer Center, our clinical proton therapy program was initiated after the development of center-specific proton therapy planning strategies, , completion of dosimetric comparison studies, , and advancements in plan-specific quality assurance measurement methods. The first HNC patient was treated at our center with passive scatter protons in 2007 and active (spot) scanning IMPT in 2010. As the number of proton therapy centers continues to expand and as clinical follow-up data continue to mature, the number of clinical studies and trials, with corresponding publication of clinical outcomes, is anticipated to increase sharply. Further, the American Society for Radiation Oncology has updated its model policy with recommendations for medical insurance coverage for proton therapy, and the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology for HNC now recognize proton therapy as a standard option for advanced conformal radiation therapy that can be considered for multiple indications.
The remainder of this chapter is organized by an anatomic HN tumor subsite, concluding with a brief section on the use of proton therapy for reirradiation of recurrent or second primary HNC. Each site-specific section reviews the (1) dosimetric advantages of proton therapy (particularly IMPT) over IMRT in terms of critical organ protection and normal tissue sparing, with illustrative case examples; (2) clinical outcomes reported to date, both in terms of disease control and toxicity reduction; (3) published comparisons of results after proton therapy with those after photon-based approaches; (4) ongoing prospective studies of proton therapy for HNC; and (5) opportunities for further improvement and future directions for the field.
Base-of-skull tumors: Chordoma and chondrosarcoma
Skull-base tumors were the first tumors of the HN region to be routinely treated with proton therapy, with the initial clinical rationale of their challenging location, their proximity to critical structures, and poor outcomes after conventional treatments owing to resistance to the doses that could be safely delivered with photon-based approaches while respecting normal tissue tolerances. Proton therapy is currently widely accepted as the preferred modality for radiation treatment of skull-base chordoma and chondrosarcoma after numerous retrospective series demonstrated substantially superior outcomes relative to historical results ( Table 13.1 ). Given the location of such tumors and their proximity to critical central nervous system structures (namely brainstem, brain, and optic pathways), the use of advanced patient setup/immobilization techniques and robust planning strategies is essential to minimize and account for biophysical uncertainties (e.g., proton range). Future directions to further improve outcomes for these and other classically radioresistant tumors include optimizing the linear energy transfer distribution of proton therapy plans and identifying ways to exploit the enhanced relative biological effectiveness (RBE) of protons at the end of their range.
| References | Patients ( n ) | Type | Site | Treatment | Outcome | Toxicity | Conclusion |
|---|---|---|---|---|---|---|---|
| Rombi et al. | 26 | Retro | Skull base and axial skeleton chordoma (19) or chondrosarcoma (7) | Surgery/biopsy, PBR using spot-scanning technique, mean dose 74 CGE for chordoma and 66 CGE for chondrosarcoma at 1.8–2 CGE per fraction | 5-y LC: 81% (chordoma), 80% (chondrosarcoma); 5-y OS: 89% and 75% | No high-grade acute or late toxicity | Spot-scanning results in excellent LC with acceptable rates of late toxicity |
| McDonald et al. | 16 | Retro | Skull-base and spinal chordoma treated with surgery (15/16) and EBRT | Retreated with PBR to 71.2–79.2 CGE | 2-y LC: 85%; 2-y CSS: 88%; 2-y OS: 80% | 3 patients with bitemporal lobe necrosis, 1 patient with CSF leak and meningitis, and 1 with ischemic brainstem stroke | Reirradiation is a feasible option for patients with recurrent chordoma of the skull base or spine |
| Ares et al. | 64 | Retro | Skull-base chordoma (42) and chondrosarcomas (22) | Surgery/biopsy followed by spot-scanning PBR; mean 68.4 CGE 1.8–2 CGE per fraction 4 days/week | 5-y LC: 81% (chordoma), 94% (chondrosarcoma); 5-y DFS: 81% and 100%; 5-y OS: 62% and 91% | 94% 5-y freedom from high-grade toxicity; 2 patients experienced grade 3–4 optic neuropathy, 2 patients experienced grade 3 symptomatic temporal lobe damage | Spot-scanning is safe with efficacy and toxicity rates similar to passive scatter |
| Rutz et al. | 10 | Retro | Skull-base and spinal chordoma (6) and chondrosarcoma (4) | Surgery followed by spot-scanning PBR; median 66–74 CGE with/without chemotherapy | 100% 3-y LC, DFS, and OS | Only grade 1 acute toxicity reported. Late toxicity in 3/10 patients: pituitary, alopecia, and radiographic brain changes and auditory changes | IMPT has similar safety and efficacy as passive scatter but may allow dose intensification |
| Noël et al. | 100 | Retro | Skull-base or cervical spine chordoma | Surgery/biopsy, combined proton-photon RT; median dose 67 CGE | 2-y LC: 86%; 4-y LC: 54%; 2-y OS: 94%; 5-y OS: 81% | 42 with late complications: 11 with vision loss, 11 with neuropsych complications, 21 with decreased hearing, 16 with pituitary dysfunction | Homogeneity of dose in the target volume is an important predictor of control |
| Munzenrider et al. | 519 | Retro | Skull base chordoma and chondrosarcoma | Surgery/biopsy followed by 66–83 CGE proton-photon RT | 5-y LRFS: 73% (chordoma), 98% (chondrosarcoma); 5-y OS: 80% and 91% | 3 patients died of brainstem injury, 8 patients had temporal lobe injury; other toxicities reported were hearing loss, cranial neuropathy, endocrinopathy | Postoperative treatment with PBR is the best management strategy for patients with base-of-skull chordoma and chondrosarcoma |
Sinonasal tumors
Building on the clinical rationale for the aforementioned skull-base tumors, many centers have implemented proton therapy as a preferred radiotherapy approach for a variety of sinonasal malignancies of various histologic types, and clinical outcomes have been encouraging ( Table 13.2 ). The largest of these single-institution studies evaluated 102 patients with tumors of various histologic subtypes, treated with or without surgery followed by postoperative or definitive combination photon-proton therapy from 1991 through 2002. With a median follow-up interval of more than 5 years for surviving patients, the local control rates after treatment that incorporated proton therapy were excellent: 95% for those who underwent complete surgical resection, 82% for partial, and 87% for those with nonresected or intact tumors. The most common pattern of recurrence was distant, occurring in about 30% of patients at 5 years. The extent of surgical resection was, unsurprisingly, associated with distant metastasis and long-term outcomes. In another study, Truong et al. reported an excellent local control rate of 86% at 2 years after high-dose (median, 76 Gy [RBE]) proton therapy for sphenoid sinus tumors, a particularly challenging tumor location.
| References | Type | Accrual | Pts ( n ) | Technique | Comp photon | CCT (%) | S (%) | Histology | Follow-Up (Median) | Outcomes | Late Toxicity |
| Resto et al. | Retro | 1991–2002 | 102 | PSPT | No | 4 | 100 | Various | 61 mo | 5-y LC: 95%, 82%, and 87%; OS 90%, 53%, and 49% for complete resection, partial resection, and biopsy only | NR |
| Nakamura et al. | Retro | 1999–2012 | 42 | PSPT | No | 26 | 0 | ENB | 69 mo | 5-OS/PFS: 100/80% for Kadish A, 86/65% for Kadish B, 76/39% for Kadish C | 6 pts with G3–4 (ipsilateral visual impairment, 3; bilateral visual impairment, 1; liquorrhea, 1; cataract, 1) |
| Russo et al. | Retro | 1991–2008 | 54 | PSPT | No | 39 | 69 | SCC | 82 mo | 5-y LRC: 73%; OS: 47% | 9 pts with G3 and 6 with G4. Mostly wound site issues (e.g., fistulas). No G5 |
| Dagan et al. | Retro | 2007–2013 | 84 | PSPT | No | 75 | 74 | Various | 32 mo | 3-y LC: 83%; NC: 94%; freedom from DM: 73.2%; OS: 68% | G3–5: overall 24%. CNS necrosis: G2 in 11%, G3 in 4%, and G5 in 1 pt. G3–4 bone or soft tissue necrosis in 7 pts. 3 pts died of Tx-related complications (G5) |
| Nakamura et al. | Pro | 2009–2011 | 26 | PSPT | No | 100 | 0 | Various | NR | 3-y OS: 58% | G4: 2 pts (osteonecrosis, retinopathy); G3: 4 pts (cataract: 2, mucositis/dermatitis: 2) |
| McDonald et al. | Retro | 2010–2014 | 14 + 26 | PSPT | Yes | 75 | NR | Various | NR | NR | More feeding tubes and more morphine used in IMRT group (but more NPC in IMRT group and more paranasal in proton group) |
| Zenda et al. | Pro | 2008–2012 | 32 | PSPT | No | 0 | 0 | Mel | 36 mo | 1-y LC: 76;3-y OS: 46%; PFS: 36% | No late G3+ toxicity reported |
| Zenda et al. | Retro | 1999–2008 | 90 | PSPT | No | 12 | 18 | Various | 57 mo | 5-y OS: 64%; PFS: 44% | Late toxicity G3 in 17 pts (19%), G4 in 6 pts (7%; encephalomyelitis infection, 2; optic nerve disorder, 4) |
| Linton et al. | Retro | 2004–2012 | 26 | PSPT | No | 0 | 77 | ACC | 25 mo | 2-y LC: 95%; OS: 93% (not previously irradiated) | Late toxicity G3 in 2 pts, G4 in 1, and G5 in 1 (after reirradiation) |
| Takagi et al. | Retro | 2002–2012 | 40 | PSPT | No | 0 | 0 | ACC | 38 mo | 5-y OS: 63%; PFS: 30%; LC: 76% | 36 G3+ events in 21 pts (26%). G+ in 24 pts, mostly osteonecrosis, G4 in 9 pts (mostly vision loss) and G5 in 3 (NP ulcers). Not separated according to proton or carbon ion therapy |
| Fuji et al. | Retro | 2006–2012 | 20 | PSPT | No | 0 | 0 | Mel | 35 mo | 3-y OS: 68%; PFS: 60% | No G3. G4 in 1 pt (optic neuropathy). No G5 |
| Demizu et al. | Retro | 2003–2011 | 33 | PSPT | No | 0 | 0 | Mel | 18 mo | 2-y LC: 71%; OS: 44% | G3–4 in 3 pts: 2 G3 (cataract, oral mucositis, and pain); 2 G4 (optic neuropathy and retinopathy) |
| Patel et al. (meta-analysis) | Retro | 1975–2013 | 286 | PSPT + CIT | Yes | Various | 38 mo | Pooled OS higher at 5 y for charged particle than for photon therapy (relative risk :1.51; 95% CI: 1.14–1.99; P = .0038) and at longest follow-up (1.27; 1.01–1.59; P = .037), as well as DFS at 5 y (1·93; 1.36–2.75, P = .0003) | |||
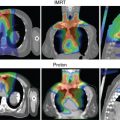
Notable findings in the studies summarized in Table 13.2 are the high rates of both local control and survival for patients with unresected mucosal melanoma after proton therapy. In general, toxicity rates for these types of tumors at this site tend to be high, reaching 20% for higher-grade toxicity; yet these rates were still comparable with those from existing photon data and were likely biased by selecting the most challenging cases to be treated with proton therapy. A meta-analysis by Patel et al. summarized a largely retrospective body of evidence and provided the strongest comparative effectiveness data for particle- and photon-based treatments for sinonasal disease. These results revealed a survival advantage for particle or proton therapy; at a median follow-up interval of about 40 months, overall survival rates were higher for those who received particle- versus photon-based treatment at 5 years (relative risk [RR]: 1.51; P = .0038) and were also higher at the longest follow-up interval (RR: 1.27; P = .037). Local-regional control rates were no different at 5 years (RR: 1.06; P = .79) but were higher for the particle therapy group at the longest follow-up interval (RR: 1.18; P = .031). The studies included in this meta-analysis were somewhat limited by the usual biases inherent in retrospective single-institution series. However, given the power and consistently favorable outcomes reported across the series, particle or proton therapy should be considered a valid option for sinonasal malignancies, and this recommendation has been incorporated into the 2017 and 2018 NCCN guidelines for HN cancer. A case study involving postoperative proton therapy for a paranasal sinus (maxillary) tumor is shown in Fig. 13.1 ; a case study of IMPT for a nasal cavity/anterior skull-base tumor that involved using an aperture to spare the anterior eyes and corneas is shown in Fig. 13.2 .