Hepatic Tumors
Laura Crocetti
Riccardo Lencioni
The development of image-guided percutaneous techniques for local tumor ablation has been one of the major advances in the treatment of liver malignancies. Among these methods, radiofrequency (RF) ablation is currently established as the primary ablative modality at most institutions. RF ablation is accepted as the best therapeutic choice for patients with very early and early stage hepatocellular carcinoma (HCC) when liver transplantation or surgical resection is not a suitable option (1,2,3). In addition, RF ablation is considered as a viable alternative to surgery for inoperable patients with limited hepatic metastatic disease, especially from colorectal cancer (CRC), in patients deemed ineligible for surgical resection because of extent and location of the disease or concurrent medical conditions (3).
Table 56.1 BCLC Classification in Patients Diagnosed with Hepatocellular Carcinoma | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
Indications
1. Very early and early stage HCC according to the Barcelona Clinic Liver Cancer (BCLC) classification (Table 56.1) when patients are not candidates for either liver resection or transplantation. Patients are required to have a single tumor smaller than 5 cm or as many as three nodules smaller than 3 cm each, no evidence of vascular invasion or extrahepatic spread, performance status test of 0, and if liver cirrhosis, Child-Pugh class A or B.
2. Nonsurgical patients with CRC oligometastases isolated to the liver. Selected patients with limited hepatic and pulmonary CRC metastatic disease may qualify provided that extrahepatic disease is deemed curable.
3. Hepatic metastases from other primary cancers. Promising initial results have been reported in the treatment of breast and endocrine tumors (3).
Contraindications
Absolute
1. Tumor located <1 cm from a major biliary duct due to risk of delayed stenosis
2. Intrahepatic bile duct dilation
3. Anterior exophytic location of the tumor, due to the risk of tumor seeding
4. Untreatable/unmanageable coagulopathy
Relative
1. Anatomic considerations
a. Greater than five lesions. The number of lesions should not be considered an absolute contraindication to RF ablation if successful treatment of all metastatic deposits can be accomplished. Nevertheless, most centers preferentially treat patients with five or fewer lesions (3).
b. Tumor size should not exceed 3 cm in longest axis to achieve best rates of complete ablation with most of the currently available devices (3).
2. Bilioenteric anastomosis, because of the risk of hepatic abscesses
3. Superficial lesions, because of a higher risk of complications
4. Superficial lesions that are adjacent to any part of the gastrointestinal tract, because of the risk of thermal injury of the gastric or bowel wall (consider hydro/gas dissection)
5. Tumors located in the vicinity of the gallbladder, due to the risk of iatrogenic cholecystitis
6. Ferromagnetic prostheses that may act as heat sinks and cause skin burns
7. Pacemaker/defibrillators. If necessary, deactivate device and reprogram.
Preprocedure Preparation
1. Evaluate patient records, history, physical examination, and prior imaging studies to determine the indication and the feasibility of ablation.
2. Preprocedural imaging—the tumor staging protocol must be tailored to the type of malignancy.
a. In patients with HCC, the detection of a nodule by ultrasound (US) is usually followed by multidetector spiral computed tomography (CT) or dynamic magnetic resonance (MR), following the recommendations of the American Association for the Study of Liver Diseases (AASLD) and of the European Association for the Study of the Liver (EASL) (1,2).
b. Patients with liver metastases should undergo abdominal US and CT or MR of the abdomen. Chest CT and positron emission tomography (PET) or PET-CT may be required to exclude or confirm extrahepatic metastatic disease.
c. Pretreatment imaging must carefully define the location of each lesion with respect to surrounding structures.
(1) Lesions located on the surface of the liver can be considered for RF ablation, although their treatment requires adequate expertise and may be associated with a higher risk of complications. Thermal ablation of superficial lesions that are adjacent to any part of the gastrointestinal tract must be avoided because of the risk of thermal injury of the gastric or bowel wall. The use of special techniques—such as intraperitoneal injection of dextrose to displace the bowel—can be considered in such instances.
(2) Treatment of lesions adjacent to the hepatic hilum increases the risk of thermal injury of the biliary tract. Thermal ablation of tumors located in the vicinity of the gallbladder has been shown to be feasible, although associated in most cases with self-limited iatrogenic cholecystitis.
(3) Thermal ablation of lesions adjacent to hepatic vessels is possible because flowing blood usually protects the vascular wall from thermal injury; in these cases, however, the risk of incomplete treatment of the neoplastic tissue close to the vessel may increase because of heat loss by convection (3).
3. Preprocedural laboratory testing
a. Measurement of serum tumor markers, such as alpha-fetoprotein for HCC and carcinoembryonic antigen for colorectal metastases
b. Evaluation of the patient’s coagulation status including measurement of the complete blood count and international normalized ratio (INR). An INR <1.5 and a platelet count higher than 50,000 per µL is required to keep the risk of bleeding at an acceptable low level.
4. Antiplatelet medications (e.g., aspirin, ticlopidine, clopidogrel, IIb/IIIa receptor antagonists) and nonsteroidal anti-inflammatory medications should be discontinued 7 to 10 days before thermal ablation. Antiplatelet therapy may be restarted 48 to 72 hours after thermal ablation.
5. Anticoagulants should be discontinued before liver ablation.
a. Warfarin should be discontinued at least 5 days before liver ablation and may be restarted the following day.
b. Heparin and related products should be discontinued 12 to 24 hours before ablation and may be restarted after 24 hours.
Procedure
1. Thermal ablation can be performed under intravenous sedation or general anesthesia with standard cardiac, pressure, and oxygen monitoring. In some centers, general anesthesia with tracheal intubation is used. The American Society of Anesthesiologists (ASA) score can be used to assess the patient’s physical status prior to RF ablation; patients with scores up to ASA III can be treated (3).
2. Technical considerations
a. RF ablation
(1) The goal of RF ablation is to induce thermal injury to tissue through electromagnetic energy deposition. With RF ablation, the patient is part of a closed-loop circuit that includes an RF generator, an electrode needle,
and a large dispersive electrode (grounding pad). An alternating electric field is created within the tissue of the patient. Because of the relatively high electrical resistance of tissue in comparison with the metal electrodes, there is marked agitation of the ions present in the target tissue that surrounds the electrode, as the tissue ions attempt to follow the changes in direction of alternating electric current. The agitation results in frictional heat around the electrode. The discrepancy between the small surface area of the needle electrode and the large area of the grounding pads causes the generated heat to be focused and concentrated around the needle electrode.
and a large dispersive electrode (grounding pad). An alternating electric field is created within the tissue of the patient. Because of the relatively high electrical resistance of tissue in comparison with the metal electrodes, there is marked agitation of the ions present in the target tissue that surrounds the electrode, as the tissue ions attempt to follow the changes in direction of alternating electric current. The agitation results in frictional heat around the electrode. The discrepancy between the small surface area of the needle electrode and the large area of the grounding pads causes the generated heat to be focused and concentrated around the needle electrode.
(2) To achieve low rates of local tumor recurrence with RF ablation, which are comparable to those obtained with hepatic resection, physicians should produce a 360-degree, 0.5- to 1-cm-thick tumor free margin around each tumor. This cuff will assure that all microscopic invasions around the periphery of a tumor have been eradicated. Thus, the target diameter of an ablation must be ideally 1 to 2 cm larger than the diameter of the tumor that undergoes treatment (3) (Fig. 56.1).
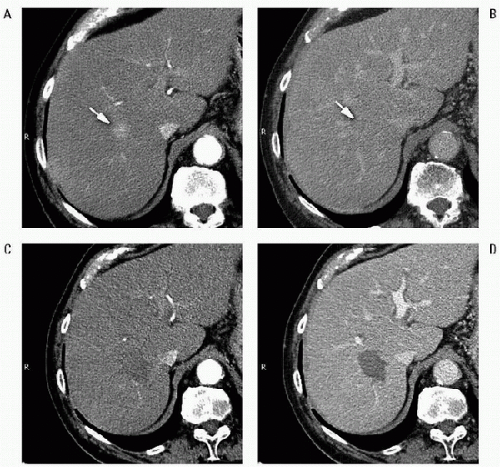 FIGURE 56.1 • RF ablation of HCC. Pretreatment CT shows the lesion as a small hypervascular nodule (A, arrow) in the arterial phase, with slightly hypodense appearance in the delayed phase (B, arrow). On CT images obtained in the arterial (C) and the portal venous phase (D) 1 month after treatment, the tumor is replaced by a nonenhancing ablation zone that exceeds in size the diameter of the naive tumor. The findings are consistent with complete response. |
(3) The thermal damage caused by RF heating is dependent on both the tissue temperature achieved and the duration of heating. Heating of tissue at 50°C to 55°C for 4 to 6 minutes produces irreversible cellular damage. At temperatures between 60°C and 100°C, near immediate coagulation of tissue is induced, with irreversible damage to mitochondrial and cytosolic enzymes of the cells. At more than 100°C to 110°C, tissue vaporizes and carbonizes. For adequate destruction of tumor tissue, the entire target volume must be subjected to cytotoxic temperatures. Thus, an essential objective of ablative therapy is the achievement and maintenance of 50°C to 100°C temperature throughout the entire target volume for at least 4 to 6 minutes. However, the relatively slow thermal conduction from the electrode surface through the tissues increases the duration of application to 10 to 20 minutes. To accomplish the increase in energy deposition into tissues, the RF output of all commercially available generators has been increased to 150 to 250 W.






