Interventional radiology (IR) has aided advances in the diagnosis and treatment of lung pathologies through procedures such as percutaneous biopsy, tumor ablation and drainage of intra-thoracic collections. The success and safety of these interventions largely depend on timely and accurate needle/device placement. Additionally, there is an inherent need to minimize radiation exposure during image-guided procedures. Robotic systems offer potential solutions to improve procedure time and accuracy, as well as reduce radiation dose. This article summarizes the existing data for clinically utilized robotic systems in the context of percutaneous lung intervention. Additionally, practical considerations are outlined when implementing robotic systems in clinical practice. Whilst robotic systems can be useful adjunctive tools, currently available systems require significant physician supervision and are therefore limited by a lack of true system autonomy.
Introduction
Interventional radiology (IR) has aided advances in the diagnosis and treatment of numerous lung pathologies. Commonly practiced IR procedures include percutaneous lung, pleural or mediastinal biopsy, ablation of lung or pleural tumors and drainage of intra-thoracic collections. Safety, technical and clinical success of these interventions are influenced significantly by timely and accurate placement of the needle/ablation device. This can be affected by physician variables, such as operator experience as well as patient variables, such as body habitus or co-operation with breathing instructions. In addition, where ionizing radiation is used for image guidance, there is an implicit imperative to reduce overall exposure.
Robotic assistance has the potential to positively assist physicians in the aforementioned domains. This article aims to outline the existing clinical evidence base relating to clinically utilized commercial robotic systems, in the context of percutaneous lung interventions. Systems are considered with regards to their overall autonomy and their integration of advanced imaging and artificial intelligence. Finally, this article aims to outline practical considerations when employing robotic systems in clinical practice.
Overview of published studies
In a systematic review of 113 percutaneous lung robot-guided biopsies, Bodard et al. showed that the technical success rates ranged from 86.7% to 100%, and diagnostic yielded between 89.4% and 100%. Two studies included a control group undergoing manual procedures. , The procedural performance for CT-guided biopsies was statistically similar between the robot-assisted and manual groups ( P = 0.05 and p-0.34, respectively). Technical accuracy, exemplified by needle positioning, showed that needle adjustments were needed less frequently in the robot-guided group compared to the manual group (2.7 ± 2.6 adjustments [range 1-4] vs. 6 ± 4 adjustments [range 2-12], P < 0.001). Both planar and craniocaudal deviations of the needle tip from the planned target were similar in both groups, measuring 2.3 ± 1.1 × 2.5 ± 1.5 mm [range 1-8] for the robot group and 3.0 ± 1.3 × 2.1 ± 1.6 mm [range 2-11] for the manual group ( P = 0.05). Two studies compared procedure time and radiation dose between robotic-guided biopsies and a control group. , Anzidei et al. showed that the robotic group experienced a 35% reduction in total procedure duration compared to the manual procedure (20.1 ± 11.3 minutes vs. 31.4 ± 10.2 minutes, P = 0.001) and a 40% reduction in radiation dose (324 ± 114.5 mGy vs. 541.2 ± 446.8 mGy, P = 0.001). However, Alexander et al. found the procedure durations were comparable between the robot and CT Fluoroscopy (CTF) groups (28 minutes [range 22-32] for the robot group and 19 minutes [range 14.3-30.5] for the CTF group, P = 0.08); radiation doses were also similar between the 2 groups, with 258 μGy·cm [range 214-372] for the robot group versus 305.5 μGy·cm [range 222-502] for the CTF group ( P = 0.39). The effective dose was also similar (3.9 mSv [range 3.2-5.6] for the robot group vs. 4.6 mSv [range 3.3-7.5] for the CTF group, P = 0.40). Regarding complication rates, Anzidei et al. reported similar rates between the robot-guided and control groups (10.4% vs. 11%, P = 0.05). Similarly, Alexander et al. reported comparable adverse events between the 2 groups ( P = 0.45), with 6.7% (1 out of 15) resulting in grade 3 pneumothorax requiring drainage in the robot group versus 12.1% (8 out of 66) in the CTF group ( P = 1).
Overview of clinically utilized robotic systems
Robotic systems have been developed for usage in percutaneous IR procedures as well as those assisting in catheter-directed vascular IR procedures. The relevant systems pertaining to the former category of intervention are considered further in this article. Such systems can be broadly divided into 2 categories: those which assist with needle positioning/tracking (and do not advance the needle towards the target) and those which can assist with needle advancement (i.e. capable of physically driving the needle towards the designated target). Similarly, systems can be patient-mounted, table-mounted or floor-mounted. Degrees of freedom (DOF) refers to the number of planes of motion the robotic arm (or equivalent) is able to move through.
Needle-positioning robotic systems
ROBIO EX and MAXIO (Perfint Healthcare Pvt. Ltd, Florence, Oregon, USA) are needle positioning floor-mounted robotic systems which have been used safely in CT-guided lung procedures in a total of 99 patients. , Both systems have 5 DOF. Once positioned, the physician advances the needle through a holder towards the intended target.
Other similar needle-positioning systems (though lacking peer-reviewed studies describing their use in lung procedures) include Micromate (Interventional Systems/iSYS Medizintechnik GmbH, Kitzbuehel, Austria), Innomotion (Innomedic, Herxheim, Germany), Needle Placement System (NPS) (DEMCON Advanced Mechatronics, Enschede, Netherlands) and EPIONE (Quantum Surgical, Montpellier, France).
Needle‑driving robots
XACT ACE Robotic System (XACT Robotics, Caesarea, Israel) is a patient-mounted robotic system which has been safely used in CT-guided lung procedures in a total of 72 patients. , The system has 5 DOF and is able to advance and the steer the needle into the designated target via a pre-planned route. However, at the time of writing, the system is no longer commercially available. Zerobot (Medicalnet Okayama, Japan) is a floor-mounted which has been used safely in CT-guided lung procedures in a total of 3 patients. The system has 6 DOF and is able to advance and steer the needle into the designated target but requires continuous physician supervision. AcuBot (Johns Hopkins University, Baltimore, Maryland, USA) is a table mounted system which can be used under CT and X-ray fluoroscopy and has been used safely in a total 2 patients. This system is based on 2 other robotic modules: RCM (remote center of motion) and PAKY (percutaneous access to kidney), representing the actuator for needle orientation and the needle driver, respectively. The system has 6 DOF and is able to advance and steer the needle towards the intended target. Neither Acubot and Zerobot are however commercially available.
Practical considerations for robotic lung interventions
The clinical evaluation of patient’s undergoing lung intervention with robotic assistance is similar to those undergoing other normal pre-procedural work up. Particular consideration should be made with regards to the robotic system being used i.e. patient-mounted, floor-mounted or table-mounted, as certain patients may not tolerate or be comfortable with a patient-mounted system. Indications and contraindications for undertaking robotic-assisted lung intervention are similar to a patient undergoing non-assisted intervention. Review of the relevant manufacturer documentation is advised to determine suitability (or off-label usage) for the desired intervention.
Appropriate pre-emptive positioning of the system in the CT or angiographic suite reduces procedural time and potential intra-procedural difficulties which can be encountered with a sub-optimally located system (e.g. making gantry access cumbersome). Needle selection is generally not limited by robotic systems and can align with operator and institutional practice, but should be confirmed with manufacturer documentation. Dependent on the robotic system being used, potential loss of usable needle length should be accounted for, to ensure the ability to advance the needle through the entirety of the planned path (without requiring disengagement with the robotic system). Future systems may incorporate software which can automatically detect, segment and plan an optimal path to a target lesion. Use of a system which can incorporate a respiratory motion sensor is preferable and where feasible needle advancement should occur in the end-expiratory phase. Where an intercostal approach is required, a path traversing the mid-rib region is suggested to minimize the risk of hemorrhage and reduce the incidence of bony deflection, particularly if there is planned angulation of the needle path.
Proactive consideration of the physical location/presence of robot is also essential in the context of managing complications. It should ensure that the system does not greatly impede the expedient necessary steps involved if encountering hemoptysis, pneumothorax, hemodynamic compromise or air embolism, requiring immediate intervention. Post-procedural workflow is identical to non-robotic procedures with the usual level of vigilance with monitoring for immediate or acute complications.
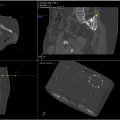
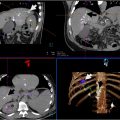
An example case of a CT-guided robot-assisted percutaneous lung biopsy is included in Figure 1 .