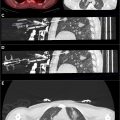
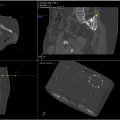
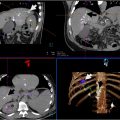
Robotic systems for minimally invasive procedures, particularly in interventional oncology, have advanced significantly, especially for percutaneous interventions guided by CT, Cone-beam CT, and MRI. These systems, which include needle-guiding and needle-driving robots, enhance the precision of procedures like biopsy and tumor ablation. Needle-guiding robots plan and align the needle, while needle-driving robots autonomously advance it, improving needle placement accuracy, enabling out-of-plane insertion, and reducing radiation exposure. These robotic systems offer key clinical benefits, such as stable needle guidance for challenging angulated approaches and better access to lesions in confined spaces, like CT or MRI gantries. They can guide the needle to the optimal region of a lesion without the need for a second contrast injection, improving both diagnosis and treatment. While many robotic systems have been developed, only a few have reached clinical use. Early studies show promising results, but concerns about increased complexity and cost remain. Further research and clinical trials are needed to fully evaluate their value, though we believe that robotic systems will play an increasingly important role in the future of image-guided interventions, particularly for challenging tumors.
Introduction
The evolution of modern medicine has increasingly favored minimally invasive techniques over traditional open surgeries. While robotic systems were first introduced in neurosurgery in the late 1980s, the field of interventional radiology (IR) has seen significant and continuous growth in both the complexity and the number of robotic-guided procedures over the past twenty years. ,
In the management of solid tumors, minimally invasive percutaneous procedures including tissue biopsy and ablation have taken essential roles and gained widespread acceptance due to their clinical effectiveness and safety, largely supplanting surgical interventions in eligible patients. Precise needle placement is required in both procedures to obtain sufficient tissue samples or achieve an adequate ablation zone. Success of IR treatments highly depends on physician expertise and accurate insertions could be challenging depending on lesion location, visualization, depth, proximity to critical structures, and patient’s breathing. This often necessitates frequent needle adjustments with repetitive imaging, leading to prolonged procedural times and increased radiation exposure for both the patient and the operator. Technical issues can result in complications or incomplete treatment, especially for tumors in difficult-to-access areas ( Table 1 , Figs. 1-7 ).
| Literature Reference (Author) | Device (Robot Name, Company Name) | Field (Technique, Image Guidance) | Features | Results Obtained |
|---|---|---|---|---|
| Bonnet et al. | EPIONE (Quantum Surgical) | CT | 6 DOF | Study of 41 patients for percutaneous ablation (RFA, MWA, IRE, cryoablation) of abdominal tumors. Technical success was achieved in 95% of patients (39/41) and 96% of needle insertions (76/79). One grade 3 complications occurred. The overall clinical success rate was 100%, with 3-month local tumor control rate of 95% (38/41). |
| Kettenbach et al. | INNOMOTION (Innomedic GmbH) | MRI | 6 DOF | Clinical trial with 12 patients demonstrated the system’s efficacy in MR-guided percutaneous biopsy, drainage, and tumor ablation in the chest, abdomen, and retroperitoneum, achieving 100% technical and clinical success with no intraprocedural complications. |
| Abdullah et al. | ROBIO™ EX (Perfint Healthcare Pvt. Ltd) | CT | 5 DOF | Study with robotic-assisted CT-guided RFA in 11 patients with 17 liver lesions. All lesions were successfully treated without RFA needle repositioning. Needle adjustments were needed in 6 lesions: 4 with 1 readjustment and 2 with 2 readjustments. Robotic planning and needle placement showed high accuracy, simplicity compared to non-robotic methods, and reduced radiation exposure for patients and staff. |
| Schaible et al. | Maxio (Perfint Healthcare) | CT | 5 DOF | Retrospective study evaluating robotic-assisted versus manual guided MWA of 368 liver tumors in 192 patients.The primary technique efficacy outcome of the group treated by robotic guidance was significantly higher than that of the manually guided group (88% vs. 76%; p = 0.013) |
| Engstrand et al. | iSYS (iSYS Medizintechnik GmbH) | CT | 4 DOF | Studied percutaneous MWA of liver tumors in 20 patients, with a median of zero antenna readjustments (range 0-1). There were no major complications, and the mean patient radiation dose was 957.5 ± 556.5 mGy × cm, with no exposure to medical personnel. |
| Heerink et al. | NPS (DEMCON Advanced Mechatronics) | CT | 6 DOF | Prospective RCT comparing robotic vs. freehand needle positioning in CT-guided MWA for liver tumors in 31 patients. Robotic arm reduced needle repositioning, improved out-of-plane target accuracy, but took longer than freehand targeting. |
| Levy et al. | XACT ACE (XACT Robotics) | CT | 5 DOF | An in-vivo study of 32 percutaneous biopsies showed that CT-guided procedures with this robotic device had a 100% success rate and higher needle placement accuracy (within 2 mm of the target) compared to standard methods. |
| Knott et al. | RAST (VortxRx; HistoSonics, Inc) | US | The study showed RAST’s feasibility in a live porcine model, using non-invasive, non-thermal focused ultrasound histotripsy for renal ablation, achieving complete histologic destruction of targeted renal tissue while preserving the urothelium. |







To address these challenges, multiple robotic navigational systems have emerged in recent years. These systems aim to alleviate the aforementioned problems by offering a precise, reproducible, time-efficient, and radiation-conscious approach to percutaneous procedures. , In addition, robotic-assisted tools also incorporate image-fusion software, enabling targeting of lesions under computed tomography (CT) guidance that may otherwise only be visible on magnetic resonance imaging (MRI), multi-phased contrast-enhanced studies, or positron emission tomography (PET)-CT scans.
Early phantom and clinical experience studies have demonstrated potential improvements in out-of-plane probe insertion accuracy and reduced learning curves for less experienced operators, along with decreased radiation exposure.
Most modern robotic systems function as navigational tools for guiding needles, assisting in planning the path, aligning it with the intended trajectory, and enabling manual insertion into the targeted tissue by physicians. Some robots also feature remote needle-driving capabilities, allowing users to plan, align, and advance the needle into the target lesion under remote guidance. Throughout this paper, we provide a comprehensive review of the technological aspects and current status in the field of image-guided percutaneous robotic interventions for solid organs.
EPIONE
The EPIONE (Quantum Surgical, Montpellier, France), is a commercially available, floor-mounted robotic system for CT-guided percutaneous needle insertion. It features 6 degrees of freedom (DOF) and includes 5 components: a mobile arm with a needle guide, an infrared navigation camera, a workstation, and a patient reference that tracks respiratory cycles. The open robotic solution is compatible with commonly used imaging systems, allowing physicians to utilize their preferred ablative technology including radiofrequency ablation (RFA), microwave ablation (MWA), cryoablation, and irreversible electroporation (IRE). After defining the tumor margin, operators select the ablative modality and number of probes. Fusion software visualizes the ablation zone coverage. EPIONE fuses MRI images with intraprocedural CT images, allowing targeting of lesions otherwise visible only on CT. The robot registers to the patient and synchronizes with the respiratory monitor, guiding the probe to the entry site. A physician advances the probe in a single pass, performing tumor ablation according to standard protocols. Postprocedural CT images can be overlaid with preoperative images to assess ablation versus tumor volumes. This system has been safely used for CT-guided percutaneous needle placement in targeting fiducials in the livers of ten swine and the kidneys of 2 swine. , All needle insertions successfully reached their targets on the first attempt without requiring readjustment, although 2 subcapsular hematomas occurred. De Baère et al. assessed the practicality and safety of the robotic system for percutaneous needle insertion during thermal ablation (RFA or MWA) of liver tumors in 21 patients. They demonstrated that robotic-assisted thermal ablation was feasible in 22 out of 23 lesions (95.7%), adjustments were unnecessary in 70.8% of tumors, and no adverse events were reported. The local tumor control rate was 83.3% for patients and 85.7% for tumors at 6 months. More recently Bonnet et al. investigated 41 patients who underwent robot-assisted CT-guided percutaneous ablation (including MWA, RFA, IRE and cryoablation) of 48 abdominal tumors. Lesions were located in the liver, kidney, adrenal gland or retroperitoneum. Technical success was achieved in 95% of patients (39/41) and 96% of needle insertions (76/79). The mean lateral distance between the needle tip and planned trajectory was 3.2 mm before adjustments and 1.6 mm after 29 manual depth and 33 lateral adjustments. Two needles required complete reinsertion. One grade 3 complication occurred (1/41; 2%). The overall clinical success rate was 100%, with a 3-month local tumor control rate of 95% (38/41). We successfully treated 76 patients (between May 5th and March 4th 2024) with MWA, IRE, and cryoablation for tumors in the liver, pancreas, kidneys, and intra-abdominal soft tissues using this robotic system. Our initial experience indicates that ablations, particularly for tumors in challenging locations, can be performed more accurately.
INNOMOTION
MRI has become increasingly popular in interventional procedures due to its excellent soft tissue contrast resolution, lack of ionizing radiation, and multimodality sensing capabilities, such as blood flow, motion, temperature, deformation and strain. One robotic system that is both CT- and MR-compatible is INNOMOTION (Innomedic, Herxheim, Germany). The INNOMOTION robotic arm features 6 degrees of freedom (DOF) and includes a module for a sleeve needle holder that provides 2 DOF in the X and Z axes, allowing for precise instrument positioning inside the magnet. Once the access trajectory is planned, the system adjusts the guiding arm, after which the physician manually inserts the needle. , The robotic arm is attached to a ring, which is then mounted onto the patient table, ensuring high accuracy. However, drawbacks include the large size of the control, which may limit interventions in confined spaces within CT or MR gantries, and less flexibility in selecting entry points compared to single-arm design solutions. The robotic system’s accuracy under MR guidance was evaluated using a porcine kidney embedded in a gelatin phantom, leading to INNOMOTION receiving CE marking for percutaneous interventions based on the findings. A clinical trial in 2008 involving 12 patients demonstrated the system’s efficacy in MR-guided percutaneous biopsy, drainage, and tumor ablation in the chest, abdomen, and retroperitoneum. All procedures were technically and clinically successful, with no intraprocedural complications reported. Another study involving 20 patients assessed MR-guided transgluteal prostate biopsy which was successfully performed with a median deviation from the needle tip to the planned position of 0.9 mm.
ROBIO
The ROBIO TM EX (Perfint Healthcare Pvt. Ltd, India) is a CE-marked robotic system compatible with CT and PET-CT. Its robotic arm offers 5 DOF movement, including 2 linear motions for guide positioning and 2 angular motions to adjust the needle’s entry angle. Designed for thoracic and abdominal interventions such as biopsy, drainage, and tumor ablation, the ROBIO also features a breath-hold management system to stabilize targets affected by respiratory movement. However, a significant drawback is that the system is fixed to the floor, requiring the needle to be decoupled whenever the CT table is moved. Anzidei et al. assessed the effectiveness of the ROBIO for CT-guided lung biopsy in 100 patients, comparing it with the conventional manual technique. Their findings demonstrated that robotic assistance significantly improved diagnostic accuracy while reducing procedure duration and radiation exposure. Abdullah et al. investigated the ROBIO system for robotic-assisted CT-guided RFA of the liver on 11 patients (17 lesions). Lesions varied in depth from 6.2 to 13.7 cm and in diameter from 1.1 to 3.0 cm. All lesions were successfully targeted using robotic assistance without the necessity for needle repositioning in any patient. Readjustments to the RFA needle were necessary in 6 lesions, with 4 lesions requiring a single readjustment and 2 lesions requiring 2 readjustments. Robot-assisted planning and needle placement show high accuracy, are technically simpler than non-robotic methods, and significantly reduce radiation exposure for both patients and staff.
MAXIO
Another robotic system developed by Perfint Healthcare is MAXIO. While both Robot EX and MAXIO are designed for image-guided procedures, they have different specifications and functionalities. MAXIO, a CT-guided, floor-mounted robotic system, is often employed for more complex procedures like MWA. It includes a stereotactic device with a 5-DOF robotic arm, multiplanar capability, software installed on a computer, and a respiratory gating system. The system provides preoperative planning assistance, intraoperative guidance, and post-procedure verification support. Once the CT volume data is transferred to the workstation, the software enables tumor segmentation, needle path planning, and ablation zone simulation with multiple RFA or MWA probes using multiplanar CT images. Mbalisike et al. evaluated the MAXIO robotic system for guiding MWA procedures. They compared it with manual approaches and found that the robotic system improved targeting accuracy, reduced patient radiation exposure, and enhanced procedural efficiency and safety during ablation. Beyer et al. conducted a comparison between conventional CT-guided manual MWA of malignant liver tumors and a robot-assisted approach utilizing the MAXIO system in 64 patients. Their study demonstrated that the robotic assistance significantly reduced procedural time and lowered the dose-length product. Moreover, robotic guidance resulted in significantly higher procedural accuracy, as measured by the deviation of needles from a defined reference (needle deviation 1.6 mm vs 3.3 mm with manual technique). Another retrospective study compared CT-guided robot-assisted MWA with manual MWA for 369 liver tumors in 192 patients, revealing a significantly higher primary technique efficacy in the robotic guidance group compared to the manual group (88% vs. 76%).
MICROMATE
Micromate (Interventional Systems/iSYS Medizintechnik GmbH, Kitzbuehel, Austria), the latest generation of robots formerly known as iSYS1 or B-Rob, is a received CE and FDA-approved, table-mounted robotic system designed for percutaneous procedures and has been utilized in both pre-clinical and clinical environments. , Compatible with cone beam CT (CBCT) as well as CT/fluoroscopy, the robot features a 4-DOF robotic positioning unit with a 2 DOF translational workspace for needle positioning in X-Y direction and an additional 2 DOF angulation of ± 32 degrees for adjusting its angle. After transferring the CT data, the trajectory is chosen, and the robotic unit moves to the correct position, depth, and angle. The needle is then manually inserted through the needle guide. In a phantom study, the iSYS1 robot successfully completed 40 needle punctures, including 20 in single and 20 in double oblique trajectories The procedures averaged 3 minutes 59 seconds, with an overall needle tip deviation of 1.1 mm (range 0-4.5 mm) from the predefined path. Another study employing the robotic system for CT-guided punctures in a torso phantom reported a mean discrepancy of 1.3 ± 1.2 mm between planned and actual needle depths. Engstrand et al. evaluated the robotic system for percutaneous MWA of liver tumors in 20 patients, with a median of zero antenna readjustments (range 0-1). There were no major complications, and the mean patient radiation dose was 957.5 ± 556.5 mGy × cm, with no exposure to medical personnel.
Needle placement system (NPS)
The needle placement system (NPS; DEMCON Advanced Mechatronics, Enschede, the Netherlands) is a table-mounted robot designed for automated orientation of the needle guide in CT-guided procedures. It features 2 stacked, rotatable segments connected to a 6-DOF locking module. The system calculates the needle trajectory and depth, aligning the needle holder with the expected entrance point, after which the operator manually inserts the needle. Needle placement precision of 1.2 to 2.1 mm of the NPS robot was demonstrated in a phantom study. Heerink et al. conducted a prospective randomized controlled trial comparing robotic versus freehand needle positioning in CT-guided MWA ablation of liver tumors in 31 patients. The study showed reduced need for needle repositioning and increased accuracy for out-of-plane targets with the robotic arm, although robotic guidance took more time than freehand targeting.
XACT ACE
The XACT ACE Robotic System (XACT Robotics, Caesarea, Israel) is a patient-mounted 5-DOF robotic system for CT-guided needle insertion. The system, which attaches to the patient via a flexible harness, advances and steers the needle based on a trajectory displayed on a workstation screen once the entry point and target are registered. The operator controls needle advancement using a foot pedal, with a respiratory sensor ensuring movement during the appropriate respiratory phase. The system also allows for needle course corrections during insertion to account for target movement, updating the trajectory based on real-time CT scans. Levy et al. assessed the accuracy, procedure duration, and radiation dose of the robotic device in 32 percutaneous abdominal and pelvic biopsies. Their findings highlighted the advantages of this device, including the ability to pre-plan the needle trajectory and make real-time adjustments without operator intervention. They achieved needle targeting with less than 2 mm error using the CT-guided robotic system. Additionally, the XACT device maintained precision despite respiratory movements, with 1.7 mm average accuracy and a procedure time under 8.5 minutes in lung ablations, enabling quicker diagnoses and treatments. The XACT system is currently not available commercially.
Robotically assisted sonic therapy
Robotically Assisted Sonic Therapy (RAST) (VortxRx, HistoSonics, Inc., Ann Arbor, Michigan, USA) is a procedure that combines histotripsy with a robotic arm and software control. Knott et al. demonstrated the feasibility of RAST in a live porcine model, utilizing non-invasive and non-thermal focused ultrasound therapy based on histotripsy for renal ablation. The study observed complete histologic destruction of the targeted renal tissue while preserving the urothelium. Unlike thermal ablation, which may be limited for tumors involving the renal hilum, histotripsy relies on cavitation—a process involving the rapid expansion and collapse of a bubble cloud—to mechanically disrupt targeted tissue using high-intensity, low-duty-cycle pulses of focused ultrasound that converge at a focal point. This binary process ensures that cavitation only occurs when a specific threshold is reached, thereby potentially avoiding damage to non-target structures. Ablations with RAST involve attaching the therapy transducer for therapeutic ultrasound and the imaging transducer for real-time ultrasound guidance to a robotic arm. These transducers are driven by micro-positioning motors capable of moving in the x, y, and z planes. The transducer position is controlled by a proprietary software package, allowing the operator to prescribe virtually any size, shape, and volume of ablation.
Discussion and conclusion
Recent advances in robotic technologies have resulted in improvements in robotic-assisted procedures. To optimize IR services, it is crucial to address key areas of concern including the economic, ergonomic, and educational aspects of new technology implementation. Advanced image guidance and robotics, despite their initial high costs, may lead to significant savings by reducing the need for repeat procedures due to increased accuracy and precision, minimizing contrast agent usage, and lowering radiation exposure. It is our experience that robotic guided ablation procedures result in faster procedures, especially for lesions in challenging locations which often require out of plane imaging guidance. Technologies such as PET and MRI provide precise imaging, improving procedural accuracy and enabling more targeted treatments. These improvements lead to better patient outcomes, reduced hospital stays, and overall long-term cost savings, making these technologies financially viable for healthcare institutions. Ultimately, with further developments in remotely controlled robotic systems, robotic-assisted IR may lead to improved access to healthcare, especially in rural areas.
Regarding ergonomic aspects, the physical demands on interventional radiologists can be alleviated by redesigning equipment and incorporating robotic systems. These advancements can help reduce procedure time, radiation exposure to the patient and operator and improve throughput. The software enhancements that allow multineedle planning and ablation confirmation can help improve workflow and outcomes.
Continuous education and training are essential for the successful integration of innovative tools in IR. Virtual reality simulation provides realistic, immersive training experiences, allowing the new generation of interventionalists to develop practical skills in a controlled environment which may lead to minimization of inter-user variability. Additionally, ongoing education through conferences, hands-on-workshops, and educational programs ensures that IRs remain updated on the latest technological and procedural advancements. This culture of continuous learning enables IRs to effectively utilize advanced image guidance and robotics, which is essential in this rapidly evolving field.
In conclusion, the combination of advanced image guidance and robotics offers significant benefits for IR. By addressing economic, ergonomic, and educational aspects, we can fully harness their potential to enhance patient care. Robotic-guided interventions, with established safety records and promising efficacy, continue to advance. While considering the balance between efficacy and cost, it is essential for IRs to lead the development in robotic interventions to maximize benefits for patients, operators, and health systems.
Patient consent
For this type of study additional formal consent is not required.
Declaration of competing interest
G. Narayanan is a Scientific Advisory Board member of Quantum Surgical.
Funding: This study was not supported by any funding.
# Contributed equally.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree