Myelopathy refers to spinal cord involvement from diverse etiologies resulting in neurologic symptoms. The presentation is variable, often posing a diagnostic challenge due to overlapping clinical and imaging features. Classification by symptom onset aids in the differential diagnosis and is based on hyperacute, acute/subacute, and chronic presentations, each carrying distinct prognoses. MR imaging is pivotal for diagnosis, providing detailed characterization of spinal cord involvement. This article offers a comprehensive overview of various diseases causing myelopathy, their imaging manifestations, differential diagnoses, and diagnostic algorithms, emphasizing the role of MR imaging in facilitating prompt identification of underlying causes and guiding optimal management.
Key points
- •
Myelopathies have diverse causes, including inflammatory, metabolic, vascular, and neoplastic diseases, necessitating thorough recognition for accurate diagnosis.
- •
MR imaging is crucial for diagnosing myelopathies, providing detailed characterization of spinal cord abnormalities and guiding treatment decisions.
- •
Compression is the primary cause of myelopathy and requires prompt evaluation with CT and MR imaging to determine its severity and guide intervention.
- •
Symptom onset, MR imaging findings, and laboratory tests aid in narrowing the differential diagnosis, while lesion distribution and extent provide additional diagnostic insights.
| ADEM | acute disseminated encephalomyelitis |
| AQP4 | aquaporin-4 water channels |
| ATM | Acute transverse myelitis |
| AVF | arteriovenous fistulas |
| AVM | arteriovenous malformations |
| CAR | chimeric antigen receptor |
| CMV | cytomegalovirus |
| CRS | cytokine release syndrome |
| CSF | cerebrospinal fluid |
| CT | computed tomography |
| DWI | diffusion-weighted imaging |
| GRE | gradient echo |
| ICANS | immune effector cell-associated neurotoxicity syndrome |
| LETM | longitudinally extensive transverse myelitis |
| MOG | myelin oligodendrocyte glycoprotein |
| MOGAD | myelin oligodendrocyte glycoprotein antibody-associated disease |
| MS | multiple sclerosis |
| NMDA | N-methyl- d -aspartate |
| NMOSD | neuromyelitis optica spectrum disorders |
| SCD | subacute combined degeneration |
| STIR | short-tau inversion recovery |
| T1WI | T1-weighted imaging |
| T2WI | T2-weighted imaging |
Introduction
Myelopathy is a term used to describe various processes that affect the spinal cord, resulting in a range of neurologic symptoms. Timely diagnosis is crucial for management but can be challenging due to variable pathogenesis and similar clinical presentations among different etiologies. A comprehensive evaluation, including clinical, imaging, and laboratory findings, is necessary to determine the underlying cause. ,
Classification of myelopathies based on the timing of symptom onset (hyperacute, acute/subacute, or chronic) provides a framework for differential diagnosis, with different underlying mechanisms and prognostic implications associated with each category. Hyperacute myelopathies are characterized by sudden onset and rapid progression, while acute/subacute and chronic forms present with insidious symptoms. Compressive etiologies, including degenerative and neoplastic conditions, are the most common causes of myelopathy. Inflammatory disorders, such as multiple sclerosis (MS), acute disseminated encephalomyelitis (ADEM), and neuromyelitis optica spectrum disorders (NMOSD), also contribute substantially to the myelopathy spectrum. Other causes of myelopathy include vascular disorders, infections, metabolic disorders, and trauma. Imaging studies, particularly MR imaging, play a crucial role in the diagnosis, providing detailed information about the spinal cord and surrounding structures and helping identify the underlying cause of myelopathy.
This article aims to delineate the clinical and imaging features of various myelopathies and provide a streamlined algorithm for differential diagnosis. Additionally, we emphasize the importance of MR imaging in elucidating key diagnostic clues, enabling timely identification of underlying etiologies and guiding appropriate management strategies and prognostication.
Terminology, Etiology, Classification, and Diagnostic Approach
Terminology
Myelopathy refers to a wide range of pathologic conditions affecting the spinal cord, presenting with various clinical manifestations ranging from hyperacute to chronic. Hyperacute myelopathies are characterized by rapid symptom progression, peaking within minutes to 4 hours of onset. In contrast, acute/subacute and chronic myelopathies develop more slowly, with acute/subacute extending over 4 hours to 21 days and chronic presenting over months to years. , ,
Acute transverse myelitis (ATM) refers to a distinct clinical presentation with bilateral spinal cord involvement leading to sensory or motor deficits. ATM does not represent a specific diagnosis but rather a syndrome that can originate from different underlying pathologies. Unlike ATM, partial myelitis is more localized, affecting only one side of the spinal cord or a specific tract, frequently observed in conditions such as MS. ,
Classification and etiology
Myelopathies can be disease-associated or idiopathic, the latter being a diagnosis of exclusion after ruling out known causes. Disease-associated myelitis includes a wide range of conditions, such as inflammatory (localized or systemic), vascular, and infectious disorders. In addition, various conditions can mimic inflammatory myelopathies, including metabolic and toxic abnormalities, radiation-induced changes, and paraneoplastic disorders.
Myelopathies can develop as primary spinal cord disorders or from conditions that affect the cord secondarily, such as extrinsic compression. They can also be classified based on the timing of clinical onset into hyperacute, acute/subacute, or chronic, which is a useful first step in the clinical workup ( Table 1 ). , ,
| Hyperacute myelopathy (over minutes to 4 h) | Spinal cord infarction |
|
| Acute/subacute myelopathies (Over 4 h to 21 d) |
|
|
| Demyelinating/inflammatory | ||
|
| |
|
| |
|
| |
|
| |
|
| |
| Neurosarcoidosis |
| |
|
| |
|
| |
|
| |
| Paraneoplastic & autoimmune myelopathy |
| |
| Chronic myelopathy (Over 3 wk to months) |
|
|
| Radiation myelitis |
| |
Algorithm for diagnosis
The diagnostic process for myelopathies typically includes a thorough evaluation of demographic information, timing of symptom onset, and a comprehensive clinical/neurologic examination. Classifying myelopathies by symptom onset offers a practical approach for differential diagnosis and determining the need for urgent intervention. Hyperacute myelopathies demand immediate imaging, typically MR imaging, to identify reversible or emergent conditions such as spinal cord infarction or trauma, and to prevent or mitigate further damage. The differential diagnosis for acute/subacute myelopathies is more extensive and includes compressive and noncompressive pathologies such as infectious, inflammatory, and neoplastic disease. Chronic myelopathies often indicate long-standing conditions such as degenerative spinal disease or metabolic disorders.
While MR imaging is the mainstay for characterizing myelopathies, computed tomography (CT) is the first-line technique in trauma patients and can help identify spinal canal stenosis due to fracture retropulsion or dislocations. CT is also the preferred modality for depicting ligamentous ossifications and other osseous causes of spinal canal stenosis. CT is helpful in evaluating postsurgical changes and may help identify other complications such as hematomas and abscesses, although its ability to detect intraspinal collections is limited without intrathecal contrast. Although CT cannot provide sufficient characterization of spinal cord parenchyma, CT myelography can help identify intrathecal pathologies resulting in myelopathy, such as dorsal arachnoid webs and arachnoid cysts.
MR imaging protocols to evaluate myelopathy should include T1-weighted imaging (T1WI), T2-weighted imaging (T2WI), short-tau inversion recovery (STIR), T2∗ gradient echo (GRE), diffusion-weighted imaging (DWI), and gadolinium-enhanced T1WI. Although myelopathic changes are difficult to visualize on noncontrast T1WI, T1 hyperintensity can sometimes be seen with hemorrhage due to the presence of methemoglobin. T2WI and the more sensitive STIR sequence usually show hyperintense signal corresponding to edema and inflammatory changes. T2∗ GRE sequences are useful for detecting hemorrhage from various causes, including trauma, underlying tumors, and vascular malformations. DWI usually demonstrates diffusion restriction in spinal cord ischemia and abscesses, whereas inflammatory myelopathies may rarely show diffusion restriction during the acute phase. ,
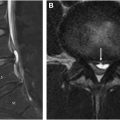
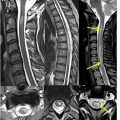
Sagittal imaging is instrumental in establishing the presence of longitudinally extensive transverse myelitis (LETM), characterized by involvement of more than 3 vertebral segments. Axial imaging is essential for differentiating between ATM, which affects most of the spinal cord cross-section, and partial myelitis, which is limited to less than 50%. , Six different MR imaging patterns of axial involvement can be described: central, centripetal, dorsal column, gray matter, anterior horn, and peripheral patterns ( Fig. 1 ). , The presence and pattern of contrast enhancement, such as nonenhancing, diffuse, patchy, or peripheral, may also be helpful in the differential diagnosis. However, lesion patterns commonly overlap, necessitating additional information such as cerebrospinal fluid (CSF) analysis and a comprehensive serum workup, including autoantibodies, metabolic, and microbiology tests. Brain MR imaging is also essential for assessing the spatial distribution of underlying pathology. Depending on the context and MR imaging findings, further studies may be required, such as body CT, PET, angiography, or ophthalmologic examination.

Hyperacute Presentation
Spinal cord infarction
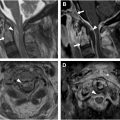
Cord ischemia is a rare cause of myelopathy, requiring a high index of suspicion for diagnosis in the appropriate clinical setting. It is characterized by an abrupt onset of symptoms that often reach their peak severity in less than 4 hours, presenting with distinctive features such as transfixing or belt-like pain, coupled with rapid and profound motor deficits. Cord ischemia can be caused by a variety of conditions, ranging from arteriosclerosis to prothrombotic states, aortic surgery/stenting, hypotension, aortic or vertebral artery dissection, and fibrocartilaginous embolism. Other rare causes include diving decompression accidents or surfer’s myelopathy due to hyperextension of the thoracic spine, also described in gymnasts. , , Ischemia most commonly affects the anterior spinal artery, which supplies a substantial portion of the central gray matter. This region relies on a limited number of vessels, particularly in the lower thoracic cord, where infarction is most common.
MR imaging may reveal anterior pencil-like stripes on sagittal imaging and an H-shaped or butterfly-shaped signal abnormality of the central gray matter on axial images, indicative of anterior spinal artery involvement. As opposed to inflammatory myelopathies, there is no appreciable cord swelling or enhancement. , , DWI is useful for revealing diffusion restriction and can show different imaging patterns, such as dorsal column involvement in posterior spinal artery infarction or a snake-eyes or owl-eyes pattern selectively involving the anterior horns of the gray matter in watershed anterior spinal artery territory infarction ( Fig. 2 ). , Associated bone marrow signal abnormalities suggestive of vertebral body infarction may be useful in challenging cases.

Acute/Subacute Presentation
Compressive myelopathy
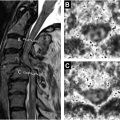
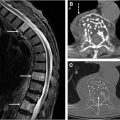
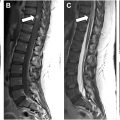
Compressive myelopathy arises secondary to mass effect on the spinal cord and leads to neurologic deficits and symptoms below the level of compression. The cervical spine is most commonly affected and patients commonly present with hand numbness, arm paresthesias, ataxia, Lhermitte phenomenon, and weakness. Compressive myelopathy is most commonly caused by degenerative spinal stenosis from disc herniations, osteophyte formation, and facet arthrosis but can also be seen with ossification of the posterior longitudinal ligament. Traumatic injuries, such as vertebral fractures or epidural hematomas, are also a common cause of spinal cord compression. Rarely, compressive myelopathy may be caused by a dorsal thoracic arachnoid web ( Fig. 3 ).


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree