Chronic thromboembolic pulmonary hypertension (CTEPH) is pulmonary hypertension secondary to chronic obstruction of pulmonary arteries by organized thromboemboli. Echocardiography and Echocardiography and ventilation/perfusion (V/Q) scan are the initial screening examinations for CTEPH; the diagnosis is often missed on computed tomography (CT). Imaging findings of chronic thromboembolic pulmonary disease overlap with those of acute pulmonary embolism, and radiologists should evaluate for the presence of concurrent chronic disease in all cases of acute pulmonary embolism detected on CT pulmonary angiography. Conditions that can mimic CTEPH include in situ thrombus, vasculitis, pulmonary artery sarcoma, and fibrosing mediastinitis.
Key points
- •
CT pulmonary angiography (CTPA) can be highly sensitive for detecting CTEPH, but the diagnosis is frequently missed by radiologists.
- •
Imaging findings of chronic thromboembolic pulmonary disease (CTEPD) differ from those of acute pulmonary embolism, though both chronic and acute disease may coexist on CTPA.
- •
Hypoperfusion can help pinpoint areas of CTEPD, a process that may be enhanced by spectral imaging techniques.
- •
Preoperative imaging aims to assess the extent of disease and identify other factors that could impact treatment decisions.
- •
Conditions that can mimic CTEPH include acute pulmonary embolism, in situ thrombus, pulmonary artery sarcoma, vasculitis, and fibrosing mediastinitis.
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is a form of pulmonary hypertension caused by prolonged obstruction of the pulmonary arteries by organized thromboemboli. Each year, approximately 600,000 Americans experience an acute pulmonary embolism. While the majority of cases resolve, about 1% to 5% result in persistent thromboembolism and CTEPH. Although a relatively rare disease, CTEPH is frequently missed both clinically and on imaging, particularly on CT pulmonary angiogram (CTPA). A recent position paper by the Fleischner Society highlighted the educational gap contributing to this underdiagnosis. CTEPH is a treatable condition with surgical, endovascular, and medical interventions that can improve both survival and quality of life, making it crucial for radiologists to recognize the imaging findings of this disease.
This article will discuss imaging of CTEPH, with particular emphasis on CTPA. We will first discuss the clinical presentation and pathophysiology of CTEPH, followed by a review of normal pulmonary arterial anatomy and CTPA technique. Finally, we will describe the imaging features of CTEPH and its mimics.
Clinical presentation and pathophysiology
The most common presenting symptoms of CTEPH are dyspnea on exertion and fatigue, which are nonspecific, likely contributing to underdiagnosis. , In the setting of right heart failure, patients may additionally experience related symptoms including lower extremity swelling, abdominal distension, chest pressure, and syncope, but these are less common presenting symptoms. Similarly, hemoptysis related to bronchial artery hypertrophy is a less-frequent presenting symptom.
Risk factors for CTEPH partially overlap with those for acute pulmonary embolism and include malignancy, chronic inflammatory states (eg, chronic infection from indwelling catheters), prior splenectomy, and hypothyroidism. While antiphospholipid antibodies and lupus anticoagulant and coagulopathies related to elevated factor VIII, von Willebrand factor, and abnormal fibrinogen are associated with CTEPH, the most common inherited thrombotic risk factors such as protein C deficiency and protein S deficiency interestingly are not.
Approximately 75% of patients with CTEPH have a history of acute pulmonary embolism, suggesting that most cases arise from the failure of acute emboli to resorb. While acute pulmonary embolism primarily consists of red blood cells, platelets, and fibrin, failure to resolve leads to infiltration by inflammatory cells, which organize the clot and deposit extracellular matrix proteins such as fibrin and elastin. This process results in a chronic thrombus that is inherently different in composition from acute pulmonary embolism. In addition to obstructive thrombi, patients with CTEPH also develop a microvasculopathy, likely due to prolonged exposure to elevated pressures and associated shear stress. In unobstructed pulmonary arteries, this is simply due to pulmonary hypertension, and in obstructed pulmonary arteries, it results from bronchial collateral vessels with systemic pressure.
CTEPH specifically refers to patients with chronic thromboembolism and pulmonary hypertension. Recent guidelines from the European Respiratory Society have proposed the term chronic thromboembolic pulmonary disease (CTEPD) to describe patients with chronic thromboembolism, with or without pulmonary hypertension at rest. However, the term chronic thromboembolic disease (CTED) is still frequently used to describe patients with chronic thromboembolism without pulmonary hypertension.
Normal anatomy
Accurate knowledge of the pulmonary arterial tree is essential for identifying and characterizing thromboembolic disease. In patients with CTEPH, occluded or diminutive pulmonary arteries can easily be overlooked if not specifically included in the search pattern.
The main pulmonary artery (PA) bifurcates into the right and left pulmonary arteries. The right PA continues as the interlobar artery beyond the first branch to the right upper lobe PA, usually the truncus anterior. Variable terminology has been used to describe the segment of the left PA distal to the first left upper lobe PA, with some still describing it as the left PA, and others using the term left descending or interlobar PA.
Segmental pulmonary arteries are named according to the bronchopulmonary segments they supply and usually run parallel to the segmental bronchi. However, many anatomic variations exist. For accurate identification of segmental pulmonary arteries, it can be useful to first locate a bronchopulmonary segment and then trace the course of its dominant pulmonary arterial supply.
The right upper lobe consists of 3 segments supplied by the apical (A1), posterior (A2), and anterior (A3) segmental arteries. The apical and anterior segmental arteries most often share a common trunk, called the truncus anterior, while the posterior segmental artery arises separately from the interlobar artery. The right middle lobe has 2 segments supplied by lateral (A4) and medial (A5) segmental branches, which may have a common trunk. The right lower lobe superior segmental artery (A6) typically originates at or proximal to the takeoff of the right middle lobe arteries. After giving off these branches, the interlobar artery continues as the right lower lobe basal trunk, which supplies the medial basal (A7), anterior basal (A8), lateral basal (A9), and posterior basal (A10) segmental arteries. The medial and anterior basal arteries (A7 + A8) usually arise from one trunk, while the lateral and posterior basal arteries (A9 + A10) arise from another.
The left upper lobe consists of the apicoposterior (A1–A2) and anterior (A3) segments, which may arise from separate segmental arteries or a common trunk. The superior (A4) and inferior (A5) lingular arteries typically share a common trunk but may arise separately. Similar to the right side, the left lower lobe superior segmental artery (A6) may arise at or proximal to the takeoff of the lingular pulmonary arteries. Beyond the A6 takeoff, the basal segmental arteries vary in their branching patterns, but most commonly, there are 2 trunks: one supplying the anteromedial basal segments (A7–A8) and the other supplying the lateral basal (A9) and posterior basal (A10) segments.
CT imaging technique
CTPA technique for CTEPH is similar to that used for acute pulmonary embolism. Typically, 75 to 100 mL of iodinated contrast is injected at 4 to 6 mL/s with adjustments based on patient body mass index. A test bolus or automated bolus triggering is used to determine the scan delay with the region of interest usually placed in the main PA. In cases of slow flow due to pulmonary hypertension or left-sided heart failure, placing the region of interest in the left atrium may help ensure better opacification of the distal pulmonary arteries. Electrocardiogram (ECG)-gating is generally not required. Scanning is performed in a caudocranial direction, as the lower lobes are more prone to motion artifacts due to greater respiratory excursion compared to the upper lobes. This approach also reduces streak artifacts caused by contrast inflow into the superior vena cava (SVC). ,
A key aspect of CTPA for CTEPH is using the thinnest possible slice thickness, typically 0.625 to 1.0 mm, to avoid the partial volume averaging that can obscure linear chronic thromboemboli. At our institution, we also reconstruct 3 plane maximum intensity projection (MIP) images at a thickness of 7 to 10 mm, which are useful for identifying small or occluded vessels.
When available, spectral imaging techniques, that is, dual-energy CT or photon counting CT, should be used for the identification of perfusion defects. At our institution, source iodine map images are used to generate 3 plane colorized postprocessed iodine maps reconstructed at a thickness of 12 mm. Although most institutions perform a single-phase CT, some have suggested the use of dual-phase CT for patients with known CTEPH to better evaluate collateral vessels, which can help differentiate acute from chronic disease. Additionally, this technique may help distinguish between artifacts such as contrast “smoke” and true thromboembolic disease.
Imaging findings/pathology
V/Q Scan
V/Q is currently the screening modality of choice to exclude CTEPD. While V/Q scan is reported to have a sensitivity of 96% to 97% and specificity of 90% to 95%, , V/Q scan may underestimate the extent of disease in cases of nonocclusive thromboembolism. When available, single photon emission computed tomography – computed tomography (SPECT-CT) is preferred over planar imaging, as planar imaging has limitations such as overlapping pulmonary segments, adjacent lung shine-through, and challenges in assessing the size of defects. ,
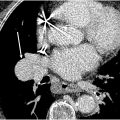
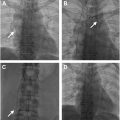
The interpretation of V/Q scans for CTEPD follows a similar approach to V/Q scans for acute pulmonary embolism, using the modified prospective investigation of pulmonary embolism diagnosis II (PIOPED II) criteria. While small mismatched perfusion defects do not exclude CTEPH, most CTEPH cases display moderate (25%–75% of a bronchopulmonary segment) or large (>75%) mismatched perfusion defects ( Fig. 1 ). When mismatches in both ventilation and perfusion are detected, further evaluation with anatomic imaging is essential to assess the full extent of the disease, evaluate lung parenchyma abnormalities, or identify potential CTEPH mimics. Several other conditions can cause vascular obstruction with preserved airflow, further discussed in the CTEPH mimics section.

CT Pulmonary Angiogram
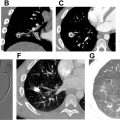
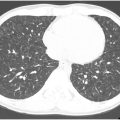
The pulmonary arterial filling defects of CTEPD on CTPA can often be distinguished from acute pulmonary embolism based on their morphology. Linear filling defects called “bands” are indicative of chronic rather than acute pulmonary embolism. Multiple bands joining together are called “webs” ( Fig. 2 A ). Chronic thromboemboli are usually eccentrically positioned within the vessel, abutting the vessel wall, in contrast to the more centrally located acute emboli ( Fig. 2 B, C). The margins of chronic emboli are usually concave, whereas acute emboli tend to have convex margins ( Fig. 3 A, B ). Consequently, chronic thromboemboli form an obtuse angle with the vessel wall, while acute emboli form an acute angle.


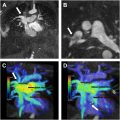
In all cases of CTEPH, occluded vessels with decreased caliber are observed ( Fig. 2 C, D). In some cases, such vessels may be so diminutive that they appear to be “absent”; in this situation, it can be helpful to use lung windows and trace the bronchi to infer the presence of occluded vessels. In contrast, pulmonary arteries affected by occlusive acute pulmonary embolism will have a normal or increased caliber ( Fig. 3 C). CT imaging can underestimate thromboembolic disease because organized thrombus may be contracted and strongly adherent to the vessel wall, making it difficult to visualize ( Fig. 4 A, B ). In fact, some cases of CTEPH will demonstrate no obvious pulmonary arterial filling defects, and it is solely the observation of reduced vessel caliber and distal occlusions, which suggests the diagnosis. This is particularly true in patients with prior splenectomy, who often only have isolated segmental and subsegmental disease ( Fig. 5 A–C ). The presence of splenectomy in a patient being evaluated for CTEPH should prompt scrutiny of subsegmental vessel caliber, even if no obvious defects are visible on initial review.



Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree