Inflammatory and infectious diseases of the temporal bone are a major indication to perform high-resolution CT and MR imaging studies. Such studies allow one to evaluate the extent of the disease in the soft tissues and in the bony structures of the temporal bone. On these same imaging studies the possible extension of the infection to surrounding regions is visualized. In this article a segmental approach is used, focusing on four structures in the temporal bone: the external ear, the otomastoid and petrous apex, the inner ear, and the facial nerve. For each of the four sections imaging findings are described and illustrated, and if relevant a differential diagnostic approach is highlighted.
Inflammatory and infectious diseases of the temporal bone are a major indication to perform high-resolution CT (HRCT) and MR imaging studies. Such studies allow one to evaluate the extent of the disease in the soft tissues and in the bony structures of the temporal bone. In most cases HRCT and MR imaging are, respectively, more indicated to do so. On these same imaging studies the possible extension of the infection to surrounding regions, such as the sigmoid sinus in the posterior fossa, or the temporal lobe of the brain, is visualized. Besides the evaluation of disease extension imaging studies are more and more used to resolve specific diagnostic challenges, such as the decision whether a cholesteatoma is present in the opacified inflammatory middle ear and mastoid, or such as the diagnostic work-up of patients suffering from long-lasting facial nerve palsy. In this article a segmental approach is used, focusing on four structures in the temporal bone (the external ear, the otomastoid and petrous apex, the inner ear, and the facial nerve), because in case of inflammatory and infectious diseases in these four regions the patient clinically presents completely differently. For each of the four sections imaging findings are described and illustrated, and if relevant a differential diagnostic approach is highlighted. Where indicated technical notes are added to the attention of the reader.
External ear
Acute external otitis is primarily of bacterial origin and is mostly seen in areas with high humidity and warm temperature. It has been associated with swimming in fresh water (swimmer’s ear) or self-inflicted trauma (eg, use of cotton-swabs). Patients with an acute infection of the external auditory canal (EAC) present with itching and pain, and sometimes otorrhea is seen. On clinical examination diffuse infection of the entire EAC or a localized furuncle is observed. More rarely external otitis is part of a viral infection by herpes zoster, and may then present with facial nerve palsy. In that case vesicles are seen in the EAC.
Almost all patients with relapsing polychondritis have ear pain and a swollen, red and tender auricle and cartilaginous EAC at first presentation. In this patient group cartilage of the nose and joints, and proteoglycan-rich structures of the eye, cardiovascular system, and inner ear also may become affected. For these infectious and inflammatory diseases of the soft tissue of the EAC the diagnosis is in most of the cases easily established by the clinical history and appearance, which makes imaging usually unnecessary.
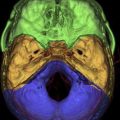
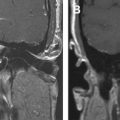
Necrotizing external otitis is considered a complication of external otitis, and is a potentially life-threatening disease because of its tendency to spread to the temporal bone and surrounding structures. This disease was initially referred to as “malignant otitis externa” because of its associated high mortality. Being an inflammatory disease rather than a neoplastic process this term has been replaced by “necrotizing external otitis.” Necrotizing external otitis is mostly seen in elderly diabetics or immunocompromised patients, although it has occasionally been reported in children or in nondiabetic, immunocompetent people. The most common pathogen of the disease is Pseudomonas aeruginosa . Fungi (mainly Aspergillus fumigatus ) may be the causative agent in nondiabetic patients. Rarely, Staphylococcus epidermidis or other gram-negative bacteria are found as an etiologic agent. The propensity of diabetics to develop necrotizing external otitis is thought to be partly caused by the compromised blood supply in diabetic microangiopathy. Additionally, the cerumen has a higher pH in diabetic patients, which might impair its bactericidal function. Pseudomonas colonize the ear canal and cause infection of the soft tissues of the EAC, which then slowly progresses to cellulitis, chondritis, and osteomyelitis. The infection extends through the fissures of Santorini and the tympanomastoid suture to the osseous EAC and subtemporal soft tissues, and spreads along vascular and facial planes to surfaces of the mastoid and petrous portion of the temporal bone, petrous apex, and skull base. In advanced cases intracranial extension can occur. The diagnosis is suspected in severe and refractory external otitis, which may be associated with neurologic symptoms or cranial nerve deficits of the facial, glossopharyngeal, vagal, or accessory nerves. On examination granulation tissue may be seen within the swollen EAC, typically located at the junction of the bony and cartilaginous portions. Cultures on the otorrhea, biopsy of the granulation tissue, and imaging studies are used for confirmation of the diagnosis, and for description of its extent. Diagnostic imaging is done with CT or MR imaging, and with radionuclide studies. In the early stage of the disease a nonspecific soft tissue swelling is present in the EAC and erosion at the petrotympanic fissure is seen. This is almost always associated with obliteration of the retrocondylar fatpad ( Fig. 1 ). The soft tissue involvement extends to the parapharyngeal space, nasopharynx, masticator space, and stylomastoid foramen. Osteomyelitis causes bone destruction of the petrous bone and skull base, including the jugular fossa ( Fig. 2 ). The infection eventually spreads to the contralateral side. Although soft tissue changes and medullary bone changes are more obvious on MR imaging, the technique does not have a higher sensitivity than CT, because cortical bone erosion, best seen on CT, is a crucial factor to establish the diagnosis. Unlike other inflammatory diseases, necrotizing external otitis shows low signal intensity of soft tissue changes on both T1- and T2-weighted images, reflecting a fibrotic necrotizing process rather than edema. Changes in the middle ear, mastoid, and medullary cavity of the basiocciput, however, show the more typical high signal intensity of inflammatory conditions on T2. After contrast administration limited enhancement of the abnormal tissues is seen. Dural enhancement may be present. In case of intracranial extension dural sinus thrombosis, signs of meningitis, or cerebral abscesses may be visualized. Radionuclide studies may help locate disease in an early stage (99mTc scintigraphy), and determine the infectious nature of the pathology (67Ga scintigraphy). Necrotizing external otitis is nowadays effectively treated with a prolonged course of antibiotics and control of diabetes. The role of surgery has become limited to biopsy and occasional surgical debridement in selected patients. Hyperbaric oxygen therapy has been recommended in advanced disease with significant skull base or intracranial involvement, and in refractory or recurrent cases. Surveillance during treatment can be done with CT or MR imaging to evaluate the soft tissue changes as a sign of response to therapy, but the bone healing lags far behind the time of successful control of the disease. Once the soft tissue involvement has disappeared surveillance should be done with 67Ga scintigraphy. With proper treatment most patients are cured and many show recovery of their facial nerve function. Children differ in presentation and response to treatment. They show a more acute and severe onset of disease. Facial nerve paralysis is more frequent, probably because of incomplete development of the mastoid and a closer position of the nerve to the EAC, and usually permanent. The tympanic membrane and middle ear are commonly involved. They respond quickly to intravenous antibiotic treatment.
Otomastoid and petrous apex
Acute and chronic infectious and inflammatory disease of the middle ear and mastoid are two distinct entities, with the former usually developing in the well-pneumatized mastoid of a child, whereas the latter is mostly seen in a more sclerotic otomastoid with a dysfuntioning eustachian tube. For that reason both entities are here discussed separately.
Acute Infectious and Inflammatory Otomastoid Disease
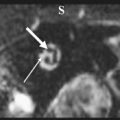
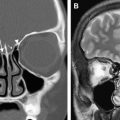
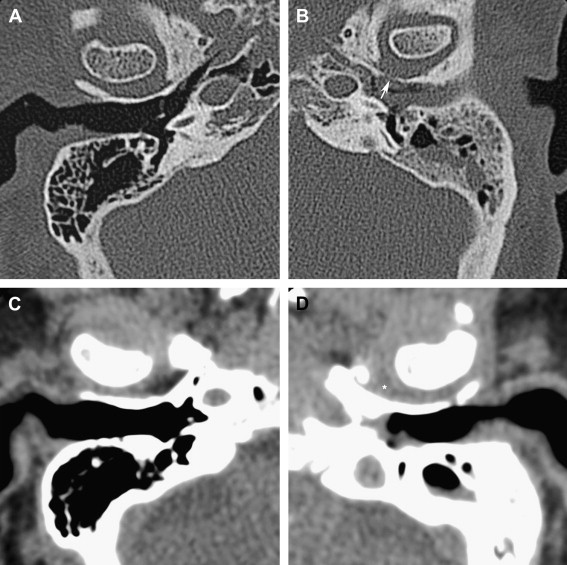
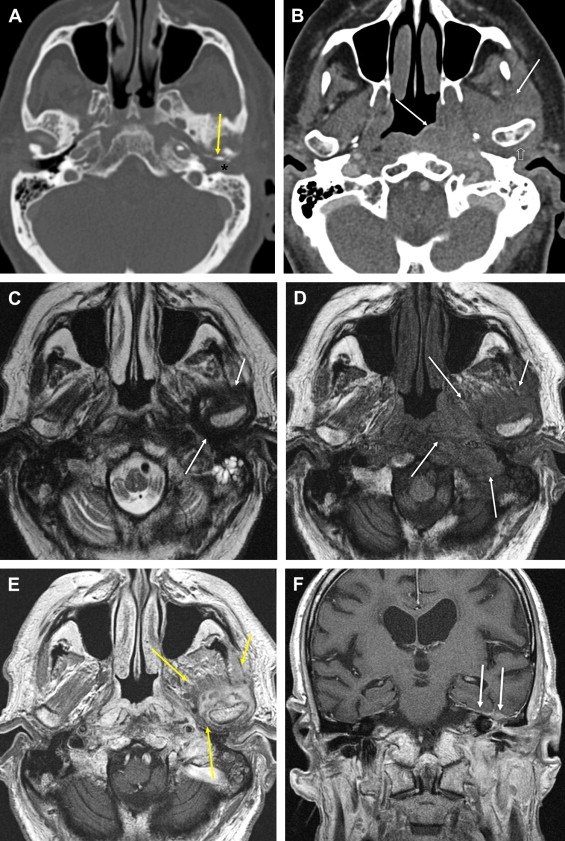
In case of mild acute otomastoid disease, most commonly caused by the pathogen Streptococcus pneumoniae , the child’s ear is painful, and the tympanic membrane is hyperemic, but an appropriate therapy can lead to healing. If the infection persists more serious complications may occur, such as coalescent mastoiditis, a condition in which mastoid cells coalesce by a process of demineralization and osteoclastic activity, or more seriously a mastoid subperiosteal abscess is formed, if the process of coalescence even extends through the cortical wall of the mastoid ( Fig. 3 ). This occurs in 50% of the patients with coalescent mastoiditis. At this stage of the disease a severely ill child presents with a postauricular soft tissue swelling and an ipsilateral, inflamed, bulging, and immobile eardrum. HRCT and MR imaging can both show this abscess. In these cases, however, CT is very important to decide whether the patient still is in the periostitis stage or whether an abscess is already present, because in this last condition more aggressive therapeutic options are considered. This CT examination demonstrates whether or not the cortical mastoid wall is intact, and in case of breakthrough enable evaluation of the extent of the soft tissue swelling. Less frequent complications of acute otomastoid disease are thrombosis of the sigmoid sinus and formation of an intracranial otogenic abscess, most frequently seen in the temporal cerebral lobe or in the cerebellum ( Fig. 4 ). Both conditions are well documented with MR imaging. In case of sigmoid sinus thrombosis phase-contrast MR angiographies and postgadolinium T1-weighted images nicely demonstrate the thrombus. Occasionally, meningitis is seen occurring after hematogeneous spread.
Chronic Infectious and Inflammatory Otomastoid Disease
In patients with chronic otitis or otomastoiditis HRCT is excellent to demonstrate the opacification of the middle ear or mastoid, but enables no differentiation between different types of effusions. HRCT moreover visualizes tympanic membrane changes, such as thickening, perforation, or retraction, but all of these are better appreciated with otoscopy. In these patients HRCT is interesting to evaluate the ossicular chain and possible causes of postinflammatory ossicular chain fixation, and to describe the status of the tympanic and mastoid walls.
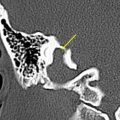
Ossicular chain erosions can be seen in both noncholesteatomatous and cholesteatomatous middle ear disease, but are far more frequent in the presence of a cholesteatoma. The malleus and incus body are much less vulnerable to this eroding process than are the incus long process, the lenticular process, and the stapes. When using correct window settings, namely a large window width (eg, 4000 HU) and a low window level (eg, 0–200 HU), CT interpretations of these smaller ossicular chain structures and their erosions are very reliable, even in an opacified cavity ( Fig. 5 ). As already mentioned, HRCT cannot differentiate between different effusions, which means that a middle ear with opacification on HRCT and demonstration of a complete ossicular chain can harbor a small cholesteatoma. Once the cholesteatoma enlarges, however, ossicular erosion and tympanic and mastoid wall erosion can occur. Concerning these wall erosions specific attention should be paid to the traditionally first affected structures: the lateral epitympanic wall (scutum); the lateral semicircular canal; the second segment (tympanic segment) of the facial nerve canal; and the roof of the middle ear cavity, also referred to as “tegmen tympani” ( Fig. 6 ).
Postinflammatory ossicular fixation is a complication of otitis media frequently seen in chronic otitis media patients with conductive hearing loss, and takes three pathologic forms: (1) fibrous tissue fixation or chronic adhesive otitis media; (2) tympanosclerosis with hyalinization of collagen; and (3) new bone formation (fibro-osseous sclerosis). In case of fibrous tissue fixation dense and permanent fibrotic adhesions occur generalized or circumscribed in the tympanic cavity. Such adhesions have a predilection for the niche of the oval window with subsequent stapedial fixation, and formation of a so-called “peristapedial tent.” When Prussak’s space is involved, fixation of the malleus head and neck results. On HRCT fibrous tissue is seen as noncalcified soft tissue debris encasing some or all of the ossicular chain ( Fig. 7 ). The diagnosis is consequently impossible on the HRCT appearance alone because of the similar density of the fibrotic tissue with other middle ear effusions or even cholesteatoma, and because fixation of ossicles cannot be determined on imaging. The differentiation on HRCT between fibrous tissue and cholesteatoma is even challenging, with cholesteatoma being more frequently associated with ossicular and tympanic wall erosions than fibrous tissue. The diagnosis of fibrous tissue fixation can be assumed if tympanic soft tissue is seen in a patient with a history of chronic otitis media with a dry ear and with conductive hearing loss. Tympanosclerosis consists of deposits of hyalinized collagen in the tympanic cavity. If it occurs in the tympanic membrane, it is also named myringosclerosis ( Fig. 8 ). Tympanosclerosis can present on HRCT in a more subtle way and is then easily missed when not compared with the contralateral side. This is illustrated by deposition of calcified debris on the stapedial crura resulting in a stapes that is “too well seen” because of its increased density ( Fig. 9 ). In more obvious conditions tympanosclerosis is characterized by unifocal or multifocal, punctate or diffuse calcified densities on the tympanic membrane, in the middle ear cavity, along tendons and ligaments, or in direct apposition to the ossicular chain. To describe the exact location of the calcified debris knowledge of the normal anatomy is mandatory, because in the middle ear cavity many prominent bone ridges and normal eminences are frequently visualized that can be confused with calcified ligaments and tendons, or with foci of tympanosclerosis. Some examples of such normal potentially confusing structures are the cochleariform process, the pyramidal eminence, and the cog ( Fig. 10 ). The cochleariform process forms a pulley over which the tendon of the tensor tympani muscle passes. The pyramidal eminence, harboring the stapedius muscle, should not be misinterpreted as a calcified stapedius tendon. The “cog” is a coronally oriented bony septum, which separates the anterior epitympanic recess from the main tympanic cavity. Even for the experienced observer, bony spurs that are frequently seen in the region of the anterior malleal ligament can be very confusing. New bone formation is the least common manifestation of postinflammatory ossicular fixation, and consists of deposition of osteoid tissue by osteoblasts on existing bony structures, leading to the formation of lamellar new bone. New bone formation is rare in the tympanic cavity but more frequently seen in the epitympanum ( Fig. 11 ). Temporal bone HRCT plays an important role in the differentiation of postinflammatory ossicular fixation from other pathologic conditions. When the tympanic membrane is intact differentiation of postinflammatory ossicular fixation from otosclerosis may be difficult clinically, because both patients typically present with a dry ear and conductive hearing loss. Temporal bone HRCT may also be helpful to evaluate the extent of the disease and predict the expected hearing improvement of surgical treatment.
CT cannot differentiate between different effusions in the middle ear and mastoid. Contrarily, MR imaging can make that differentiation. Initial reports showed that contrast-enhanced MR imaging could discriminate between primary acquired cholesteatoma and associated infection, by showing the nonenhancing cholesteatoma in the associated enhancing inflammatory tissue. On diffusion-weighted MR imaging (DWI) sequences of a cholesteatoma shows hyperintense on the B 1000 images, whereas inflammation does not ( Fig. 12 ). If in such patients a fistula is present to the lateral semicircular canal enhancement of the membranous labyrinth can also be noted, and signal loss within the perilymphatic and endolymphatic spaces on the thin-sliced heavily T2-weighted sequences. In case of tegmen disruption meningeal enhancement and signal changes in the adjacent cerebral temporal lobe tissue can be present.
In a postoperative setting after cholesteatoma surgery HRCT examinations of the temporal bone have a high negative predictive value in patients with a well-aerated middle ear. If in such well-aerated ears an isolated nodular soft tissue lesion is visualized HRCT can confirm the diagnosis of a recurrent cholesteatoma; if such a nodule is not seen, no recurrence is present. Unfortunately, most patients investigated after cholesteatoma surgery have a total or subtotal soft tissue opacification of the middle ear, and as mentioned before HRCT cannot differentiate between the different effusions, such as inflammation or abscess ( Fig. 13 ), scar tissue or granulation tissue, cholesterol granuloma ( Fig. 14 ), or even cholesteatoma. Recently, MR imaging has been suggested as a useful tool for this differential diagnosis, and on this matter two different imaging protocols have been investigated. These two protocols are based on performing either delayed postgadolinium T1-weighted imaging or DWI. The technique of delayed postgadolinium T1-weighted imaging was originally described by Williams and colleagues, and is based on the fact that cholesteatoma does not enhance, whereas inflammatory, granulation, and scar tissue does. Respecting a delay of 45 minutes after injection is mandatory to let the scar tissue enhance, otherwise a false-positive diagnosis of cholesteatoma can be made. Although DWI has originally been described by many others for its well-known use in the diagnosis of ischemic brain lesions, Fitzek and coworkers was one of the first to report that cholesteatoma shows hyperintense on echoplanar DWI, probably because of a T2-shine through effect. Recent publications have now discussed more extensively the value of echoplanar DWI in the diagnosis of primary acquired middle ear cholesteatoma, residual, and recurrent cholesteatoma. Using echoplanar DWI cholesteatomas as small as 5 mm can be visualized, but for smaller lesions, which especially residual cholesteatomas often are or also early recurrences, the resolution is too low, slice thickness too high, and moreover susceptibility artifacts of this sequence disable reliable visualization. These artifacts are most prominent at the tegmen tympani. Non–echoplanar-based DWI sequences, turbo or fast spin echo based, have a higher resolution, generate thinner slices, and do not suffer at all from susceptibility artifacts, and enable visualization of cholesteatomas as small as 2 mm. Although HRCT is worldwide still in use to image pre–second-look patients, early results indicate that the combined use of delayed postgadolinium T1-weighted images and non–echoplanar-based DWI sequences has the highest sensitivity and specificity to detect residual cholesteatoma, which can lead to an important reduction of unnecessary second-look procedures.