This review will describe various disease processes resulting in pulmonary vasculitis. The clinical and imaging findings in these diseases often overlap with diffuse alveolar hemorrhage secondary to pulmonary capillaritis, a common manifestation in many of these diseases. A multidisciplinary approach is important for the correct diagnosis of these diseases, and this review will highlight the important imaging findings that radiologists need to be aware of to aid in this diagnostic process.
Key points
- •
The pulmonary vasculitides are a heterogenous class of diseases that are associated with inflammation of the pulmonary blood vessel walls.
- •
Diffuse alveolar hemorrhage (DAH) is a common manifestation of pulmonary vasculitis and should be considered in patients with hemoptysis and imaging findings of diffuse ground-glass opacities or consolidation that do not improve with treatment for heart failure or infection.
- •
In evaluating patients with suspected pulmonary vasculitis, understanding the typical and atypical imaging appearances of common pulmonary vasculitides and reporting the pertinent positive and negative imaging findings can aid in diagnosis and classifying the patient using recent criteria.
Introduction
Introductory Case
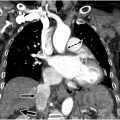
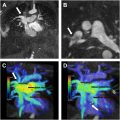
A 38-year-old female with a history of rheumatoid arthritis arrived at the Emergency Department with acute chest pain. Her electrocardiographic findings were consistent with an anterior ST elevation myocardial infarct. She was emergently taken for catheter angiography, which showed 95% stenosis of the distal left anterior descending coronary artery and was treated with a stent. A computed tomography (CT) angiogram (CTA) of the neck, chest, abdomen, and pelvis at the time of admission showed focal thickening of the left subclavian artery causing severe stenosis along with multifocal narrowing and irregularity of the right vertebral artery ( Fig. 1 ). These findings were not present on a neck CT performed 2 years earlier. Arterial wall thickening and narrowing of the bilateral iliac and femoral arteries were present and unchanged. Her initial blood work was significant for elevated erythrocyte segmentation rate and C-reactive protein.

The interpretation of the CTA of the neck favored atherosclerotic disease over vasculitis, while the CT angiogram of the chest, abdomen, and pelvis suggested vasculitis as the underlying cause. This study highlights the difficulty that can arise in assessing for vasculitis and the need to evaluate the complete clinical, radiologic, and often histologic pattern in patients with suspected vasculitis. This case will be discussed further in the concluding comments.
Background
The pulmonary vasculitides are a heterogenous class of diseases that are associated with inflammation of the pulmonary blood vessel walls. The different forms of vasculitis can be classified based on a variety of factors including pathogenesis, type of inflammation, distribution of organ involvement, and clinical manifestations. The 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides provides the most frequently used system of nomenclature for the various vasculitides. These diseases are first divided based on the size of the vessels that are most affected. Large vessel vasculitis (LVV) is primarily caused by inflammation of the aorta and its main branches. Medium vessel vasculitis (MVV) refers to inflammation of the arterial branches supplying major visceral organs like the kidneys. Small vessel vasculitis (SVV) involves the arterioles, capillaries, and venules. A few diseases have no predominant vessel involvement and fall under the category of variable vessel vasculitis (VVV). Many of the vasculitides are characterized by the presence of antineutrophil cytoplasmic antibodies (ANCA). Further classification can be made based on the distribution of organ involvement and clinical characteristics including age of onset.
In 2022, the American College of Rheumatology and the European Alliance of Associations for Rheumatology (ACR/EULAR) published new criteria for the classification of some of the large and small vessel vasculitides. Several of these new criteria include imaging findings as part of the classification system, so it is important for radiologists to be aware of what findings need to be reported to aid in the classification of these disease processes. It is important to note that these criteria are used for disease classification and have not been validated solely for the diagnosis of any vasculitis. A diagnosis of a particular vasculitis should be based on all available clinical, radiologic, and pathologic information.
The purpose of this review is to give an overview of diseases that affect the pulmonary vasculature and to focus on the characteristic imaging findings associated with each type of vasculitis. This review will discuss some of the LVV and SVV. MVV, infectious vasculitis, vasculitis associated with systemic diseases (eg, rheumatoid arthritis and sarcoidosis), and vasculitis associated with probable etiologies (eg, cancer and hepatitis B and C) will not be discussed.
Pathophysiology
The pulmonary vasculitides are usually a manifestation of a broader systemic vasculitis, although they rarely can occur as a limited vasculitis confined to the respiratory system. Along with direct vessel wall inflammation, pulmonary involvement in vasculitis can occur through 3 mechanisms:
- •
Pulmonary capillaritis resulting in DAH
- •
Inflammatory cell infiltration of the lung parenchyma often resulting in necrosis or granuloma formation
- •
Inflammation of the tracheobronchial tree resulting in airway thickening, nodularity, stenosis, or some combination.
Most pulmonary vasculitides involve the smaller vessels, primarily capillaries. Involvement of the bronchial arteries and pulmonary arteries and veins can occur in LVV and VVV but is less common than involvement of the aorta and its larger branches.
The SVV are divided into immune complex and ANCA-associated vasculitides (AAV). In immune complex vasculitis, there is marked deposition of immune complexes in the affected vessel walls. In AAV, there is usually limited or no deposition of immune complexes in the vessel walls, and instead these diseases are characterized by the presence of antibodies directed against cytoplasmic components of neutrophils and monocytes.
Imaging Findings
Multiple imaging examinations are used in the assessment of pulmonary vasculitis. The advantages and disadvantages of each modality along with common imaging findings are discussed in Table 1 .
| Modality | Advantages | Disadvantages | Common Imaging Findings Seen in Pulmonary Vasculitis |
|---|---|---|---|
| Chest radiography |
|
|
|
| Catheter angiography |
|
|
|
| Ultrasound |
|
|
|
| Computed tomography (CT) |
|
|
|
| Magnetic resonance imaging (MRI) |
|
|
|
| FDG- Positron emission tomography (PET) |
|
|
|
Pathologically, pulmonary vasculitis most commonly affects the small pulmonary capillaries. Capillaritis, the inflammation of pulmonary capillaries, leads to the destruction of the alveolar-capillary membrane allowing for alveolar leakage of red blood cells. The resulting DAH is one of the most common pulmonary manifestations of pulmonary vasculitis.
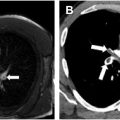
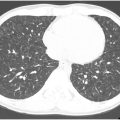
The classic radiologic appearance of DAH is bilateral ground-glass opacity (GGO) often with subpleural sparing ( Fig. 2 ). Radiographs can be normal with DAH, making chest CT the imaging modality of choice. In the acute setting, diffuse GGO is often accompanied by interlobular septal thickening secondary to lymphatic congestion from hemosiderin laden macrophages resulting in a “crazy-paving” pattern. The GGO can also coalesce into areas of consolidation (3). Consolidation can also be seen secondary to pulmonary infarcts. Organizing pneumonia near areas of hemorrhage resulting in a “reverse halo” can occur with some vasculitides. , Resolution of the radiologic abnormalities often lag behind the clinical resolution of symptoms. Granulomatous inflammation of the lung parenchyma commonly results in pulmonary nodules and masses, which can cavitate. Recurrent lung injury from repeated episodes of vasculitis can result in pulmonary fibrosis. Imaging is also important to exclude other potential causes of hemoptysis including bronchiectasis or malignancy.

Involvement of the upper and lower airways is common in many of the pulmonary vasculitides, and the pattern of airway involvement can help distinguish among different types of vasculitis. Upper airway involvement can lead to nasal obstruction and recurrent episodes of sinusitis. Repeated insults to the nasal passages can result in nasal septal destruction and collapse with saddle nose deformity. Oral ulcerations and gingival hyperplasia can also occur.
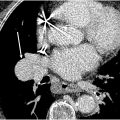
Acute and chronic airway inflammation from vasculitis can result in lower airway thickening and narrowing, most commonly involving the subglottic region ( Fig. 3 ). Focal granulomatous inflammation can result in inflammatory nodules or pseudotumors. Tracheal webs and membranes can form. CT acquired at full inspiration can underestimate the degree of lower airway narrowing. Tracheobronchomalacia can also develop secondary to airway involvement of vasculitis. Therefore, CT acquisitions in full inspiration and expiration may be useful in the initial assessment of suspected pulmonary vasculitis.

Vessel wall inflammation also occurs in MVV and LVV. On CT, the vascular abnormalities appear as vessel wall thickening, aneurysms, narrowing, and beading. MRI can add additional information with active inflammation characterized by increased signal on T2-weighted and short tau inversion recovery sequences and vessel wall enhancement on post-contrast T1-weighted sequences.
Small vessel vasculitides
Antineutrophil Cytoplasmic Antibodies-associated Vasculitides
These diseases are associated with circulating antibodies against components found in the cytoplasm of neutrophils and monocytes. The presence of ANCA is useful in the diagnosis and classification of the 3 AAVs: granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and eosinophilic granulomatosis with polyangiitis (EGPA). AAVs are the most common cause of pulmonary-renal syndrome consisting of both DAH and rapidly progressive glomerulonephritis. AAVs account for approximately 70% of patients with pulmonary-renal syndrome. It is important to consider these diseases any time a patient presents with both lung abnormalities and renal impairment.
The presence of ANCA has been reported in up to 96% of GPA patients with severe disease and 83% in limited disease with most ANCA-positive patients reactive to c-ANCA/PR3-ANCA. Up to 75% of patients with MPA are ANCA positive, the majority of which are perinuclear (p)-ANCA/myeloperoxidase (MPO)-ANCA reactive. With EGPA, ANCAs are detected in approximately half of patients with the majority of those reactive to p-ANCA/MPO-ANCA.
Granulomatosis with polyangiitis
GPA, referred to as Wegener granulomatosis until 2011, is the most common of the AAVs, although incidence and prevalence among the AAVs has considerable geographic variation. , GPA equally effects men and women. The classic clinical triad includes upper airway involvement, lower respiratory involvement, and glomerulonephritis. However, GPA can involve multiple other organs including the skin, eyes, heart, nervous system, muscles, and joints. Histologically, GPA is characterized by extravascular granulomatous inflammation along with necrotizing vasculitis affecting small and medium sized vessels leading to vascular thrombosis and tissue necrosis. , Most patients respond well to treatment, which typically involves an induction phase using immunosuppressive drugs, commonly cyclophosphamide, with or without corticosteroids, followed by long-term immunosuppressive maintenance therapy for disease remission.
The characteristic radiographic abnormality in GPA patients with active disease is multiple pulmonary nodules and masses, which often cavitate ( Fig. 4 ). CT is the preferred imaging modality for the detection of cavitary and noncavitary nodules. One study of 30 patients found nodules in approximately 90% of patients at initial presentation. In this study, 85% of patients had multiple nodules with bilateral nodules in 67% of patients. These nodules favored subpleural predominance over a peribronchovascular distribution. The nodules often coalesce and can often be secondarily infected. The halo sign can be seen secondary to hemorrhage adjacent to nodules in up to 15% of patients. An organizing pneumonia pattern of lung injury has been reported in GPA GGO and consolidation can also occur in the setting of active vasculitis and capillaritis leading to DAH.