Tumoral lesions of the temporal bone are relatively rare. Cross-sectional imaging plays an important role in the description of extension of these lesions. In certain lesions, imaging characteristics are rather specific, giving a clue to diagnosis. The most common tumoral lesions of the external, middle, and inner ear are discussed. Some rare lesions are also highlighted.
This article presents an overview of tumoral lesions of the temporal bone. These lesions are rather rare. Imaging plays a crucial role in describing the exact extent of these lesions and can be helpful in specifying some of these lesions. Classification of these tumors can be made based on location and origin, age of the patient, histologic findings, and benign or malignant aspect of the lesion. Specific attention should be paid to the young age group, because several tumoral entities are specific for this age group. Special attention should also be paid to various types of pseudolesions and benign lesions mimicking tumoral lesions. These lesions should definitely not be “touched,” because any treatment might be harmful to the patient.
In this article, classification of tumoral lesions is made based on location of the lesion: external ear, middle ear, or petrous bone/membranous labyrinth. The most common tumoral lesions are discussed. Several more rare entities are highlighted also. Petrous bone apex lesions are not discussed because they are the subject of a separate article in this issue.
Imaging
Technical Aspects
For the evaluation of a suspected tumor of the temporal bone, CT and MR imaging are used.
CT is used to evaluate the osseous invasion by the tumor. The aspect of bone invasion can provide additional information regarding the type of tumor.
MR imaging has superior contrast resolution. Various pulse sequences can be used. The use of intravenously administered gadolinium is highly recommended to visualize the enhancement of tumor, soft tissues, meninges, and surrounding vascular structures.
High-Resolution CT
Multidetector CT is used to acquire a volume data set from which multiple reconstructions can be made in different planes and in various reconstruction thicknesses. Contrary to CT performed for conductive hearing loss, CT performed for suspicion of a tumoral lesion should always be conducted using intravenously administered iodinated contrast medium. The data set should be acquired in soft tissue and by means of a high-resolution bone algorithm to allow evaluation of the soft tissues and the bony details of the temporal bone. Usually, submillimetric acquisition is performed. Multiplanar reconstructions can give additional information regarding the exact extension of the tumor and the invasion of vascular structures, nerve channels, and the membranous labyrinth.
MR Imaging
MR imaging of the temporal bone should be performed using a dedicated multichannel head coil and a high field strength MR imaging system. The authors use a combination of a multichannel head coil with small surface coils placed inside. Depending on the region to be evaluated, the head coil or small surface coils can be switched on. Both temporal bones should be imaged to compare both sides. The entire skull base should be included. Evaluation of intracranial extension can thus be done also.
An MR imaging protocol of the temporal bone should start with an entire brain examination using a T2-weighted fast spin echo (FSE), turbo spin echo (TSE) sequence, or fluid-attenuated inversion recovery sequence to exclude associated brain pathologic processes. A heavily T2-weighted sequence, usually a submillimetric three-dimensional (3D) TSE/FSE T2-weighted sequence, is performed to evaluate the fluid content and signal intensity characteristics in the membranous labyrinth. T1-weighted sequences are performed before and after intravenous administration of gadolinium: a 1-mm (or even submillimetric) axial 3D T1-weighted gradient echo sequence or a thin-slice (2 mm or less) axial and coronal SE T1-weighted sequence can be used. Fat saturation techniques should be applied in one direction after contrast administration. The acquisition matrix should be at least 512 × 512, with a field of view of 20 cm or less.
With the advent of 3-T machines in clinical practice, the axial submillimetric 3D T1-weighted gradient echo sequence is likely to become the sequence of choice because it gives the maximum number of slices through the temporal bone.
Magnetic resonance angiography (MRA) sequences are obligatory in case of a suspected glomus tumor or in the evaluation of a patient with pulsatile tinnitus. Diffusion-weighted imaging (DWI), preferably a non–echo planar imaging (EPI) DWI sequence, should definitely be added in case of a suspected tumoral or pseudotumoral lesion of the temporal bone. Using DWI, differentiation between congenital or acquired cholesteatoma and epidermoid cysts can easily be made.
Tumor Extension
Spinocellular (SCC) and basocellular carcinoma of the external auditory canal (EAC) and adenoid cystic carcinomas have the propensity to spread along the course of the facial nerve ( Fig. 1 ). The stylomastoid foramen should always be included in the scanning volume to evaluate its fatty content within the central hypointense facial nerve. Adenoid cystic carcinomas have a propensity for skip metastases; thus, careful evaluation of the entire course of the facial nerve is mandatory.
External ear and external auditory canal
Cholesteatoma and Keratosis Obturans
Cholesteatoma and keratosis obturans have been regarded in the past as the same disease process or variations of the same underlying condition. These two conditions are distinct disorders, however, with their own clinical presentations, physical and pathologic findings, and treatment.
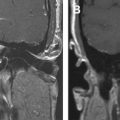
Cholesteatomas are most frequently found in the middle ear and mastoid cavity. In rare cases, they originate in the EAC. Typically, this lesion presents in the older age group, is usually unilateral, and is accompanied by a history of chronic ear discharge and otalgia. Several possible causes, such as prior surgery and trauma, have been mentioned. Congenital and acquired cholesteatomas have been reported in cases of congenital stenosis or atresia ( Fig. 2 ). Usually, external ear canal cholesteatoma requires surgical intervention to remove the cholesteatoma sac and adjacent necrotic bone. Imaging findings are nonspecific, showing a soft tissue mass in the EAC with associated bony erosion on CT. Usually, the margins of the bony erosion are sharp and regular. In case of associated periostitis, the margins become irregular. In these cases, differential diagnosis from a malignant tumor is difficult. It is known that accumulated keratin in a cholesteatoma sac causes clear hyperintensity on B1000 DWI MR imaging; thus, non-EPI DWI sequences are mandatory to make the diagnosis.
Keratosis obturans occurs in a younger patient group, often in patients with a history of sinusitis or bronchiectasis. Chronic ear discharge is rare. Symptoms are acute severe otalgia and conductive hearing loss. Contrary to cholesteatoma, keratosis obturans is often bilateral. Keratosis obturans more often causes diffuse widening of the EAC and less bony erosion.
Exostoses and Osteomata
Benign bony tumors of the EAC are exostoses and osteomas. Exostoses are far more common than osteomas.
Exostoses are broad-based lesions in the medial half of the EAC near the tympanic annulus. There is a clear relation between exostoses and a history of repetitive exposure to cold water, such as in surfing, swimming, and diving. This entity is often referred to as “surfer’s” or “swimmer’s” ear ( Fig. 3 ). They are often bilateral and asymptomatic; occasionally, they become large enough to cause obstruction of the EAC with retention of debris. CT demonstrates the broad-based bony lesion nicely and shows the extent of the disease and eventual retro-obstructive pathologic findings in the middle ear. MR imaging makes little contribution apart from differentiating such retro-obstructive soft tissue pathologic findings as cholesteatoma and inflammation.
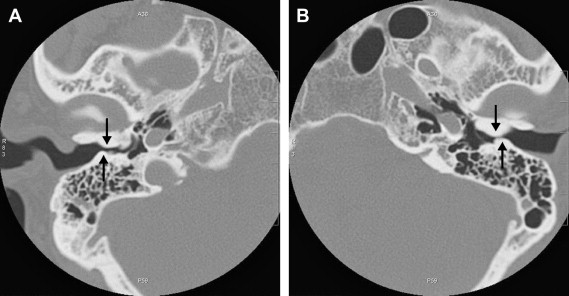
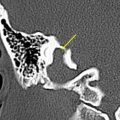
Osteomas are more rare and are situated in the lateral part of the EAC, and they are mostly unilateral. They are layered as normal bone, including central bone marrow. Osteomas may also arise in the middle ear and mastoid ( Fig. 4 ), and even in the petrous bone pyramid near the porus of the internal auditory canal (IAC).
Malignant Tumors of the External Auditory Canal
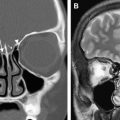
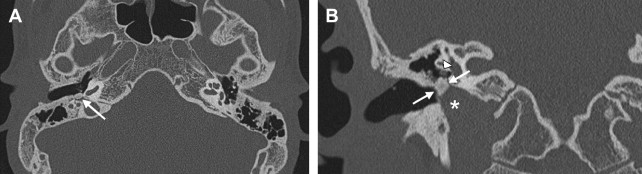
Malignant tumors of the EAC are rare. The most frequently encountered primary malignant neoplasms of the EAC are SCC ( Fig. 5 ), basal cell carcinoma, adenoid cystic carcinoma, and ceruminous gland carcinoma. SCC of the EAC and external ear is usually seen in patients with a prior history of therapy-resistant chronic external and middle ear infection. It is a tumor of the older generation (50–70 years of age).
Often, these tumors have a locally aggressive course, with extensive bone destruction on CT. MR imaging is superior in demonstrating exact soft tissue extension.
The most frequently encountered secondary neoplasms are metastatic lesions in the EAC or parotid adenoid cystic carcinoma directly invading the EAC. Breast carcinoma is the most common hematogenous metastatic lesion to occur within the temporal bone. Malignant melanoma has been reported to arise on the external ear, secondarily invading the EAC.
The prognosis of primary malignant tumors of the EAC depends on the local invasion and the extent of the disease, which can be determined by CT and MR imaging. Currently, there is no staging system for EAC tumors accepted by American Joint Committee on Cancer (AJCC) of the International Union Against Cancer (UICC). Most frequently, the Pittsburgh staging system is used.
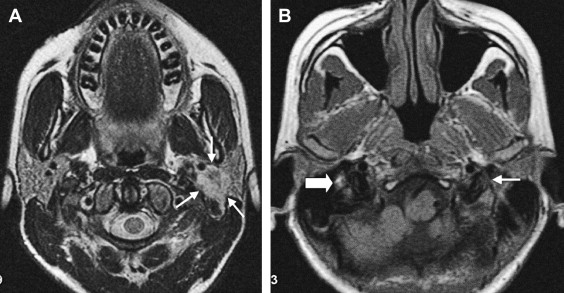
When tumors become large, the exact origin of the lesion may be difficult to determine. When extending into the temporomandibular joint and deep spaces of the neck, differentiation from necrotizing external otitis on imaging can be difficult ( Fig. 6 ). Clinical findings, however, differ completely.
External ear and external auditory canal
Cholesteatoma and Keratosis Obturans
Cholesteatoma and keratosis obturans have been regarded in the past as the same disease process or variations of the same underlying condition. These two conditions are distinct disorders, however, with their own clinical presentations, physical and pathologic findings, and treatment.
Cholesteatomas are most frequently found in the middle ear and mastoid cavity. In rare cases, they originate in the EAC. Typically, this lesion presents in the older age group, is usually unilateral, and is accompanied by a history of chronic ear discharge and otalgia. Several possible causes, such as prior surgery and trauma, have been mentioned. Congenital and acquired cholesteatomas have been reported in cases of congenital stenosis or atresia ( Fig. 2 ). Usually, external ear canal cholesteatoma requires surgical intervention to remove the cholesteatoma sac and adjacent necrotic bone. Imaging findings are nonspecific, showing a soft tissue mass in the EAC with associated bony erosion on CT. Usually, the margins of the bony erosion are sharp and regular. In case of associated periostitis, the margins become irregular. In these cases, differential diagnosis from a malignant tumor is difficult. It is known that accumulated keratin in a cholesteatoma sac causes clear hyperintensity on B1000 DWI MR imaging; thus, non-EPI DWI sequences are mandatory to make the diagnosis.
Keratosis obturans occurs in a younger patient group, often in patients with a history of sinusitis or bronchiectasis. Chronic ear discharge is rare. Symptoms are acute severe otalgia and conductive hearing loss. Contrary to cholesteatoma, keratosis obturans is often bilateral. Keratosis obturans more often causes diffuse widening of the EAC and less bony erosion.
Exostoses and Osteomata
Benign bony tumors of the EAC are exostoses and osteomas. Exostoses are far more common than osteomas.
Exostoses are broad-based lesions in the medial half of the EAC near the tympanic annulus. There is a clear relation between exostoses and a history of repetitive exposure to cold water, such as in surfing, swimming, and diving. This entity is often referred to as “surfer’s” or “swimmer’s” ear ( Fig. 3 ). They are often bilateral and asymptomatic; occasionally, they become large enough to cause obstruction of the EAC with retention of debris. CT demonstrates the broad-based bony lesion nicely and shows the extent of the disease and eventual retro-obstructive pathologic findings in the middle ear. MR imaging makes little contribution apart from differentiating such retro-obstructive soft tissue pathologic findings as cholesteatoma and inflammation.
Osteomas are more rare and are situated in the lateral part of the EAC, and they are mostly unilateral. They are layered as normal bone, including central bone marrow. Osteomas may also arise in the middle ear and mastoid ( Fig. 4 ), and even in the petrous bone pyramid near the porus of the internal auditory canal (IAC).
Malignant Tumors of the External Auditory Canal
Malignant tumors of the EAC are rare. The most frequently encountered primary malignant neoplasms of the EAC are SCC ( Fig. 5 ), basal cell carcinoma, adenoid cystic carcinoma, and ceruminous gland carcinoma. SCC of the EAC and external ear is usually seen in patients with a prior history of therapy-resistant chronic external and middle ear infection. It is a tumor of the older generation (50–70 years of age).
Often, these tumors have a locally aggressive course, with extensive bone destruction on CT. MR imaging is superior in demonstrating exact soft tissue extension.
The most frequently encountered secondary neoplasms are metastatic lesions in the EAC or parotid adenoid cystic carcinoma directly invading the EAC. Breast carcinoma is the most common hematogenous metastatic lesion to occur within the temporal bone. Malignant melanoma has been reported to arise on the external ear, secondarily invading the EAC.
The prognosis of primary malignant tumors of the EAC depends on the local invasion and the extent of the disease, which can be determined by CT and MR imaging. Currently, there is no staging system for EAC tumors accepted by American Joint Committee on Cancer (AJCC) of the International Union Against Cancer (UICC). Most frequently, the Pittsburgh staging system is used.
When tumors become large, the exact origin of the lesion may be difficult to determine. When extending into the temporomandibular joint and deep spaces of the neck, differentiation from necrotizing external otitis on imaging can be difficult ( Fig. 6 ). Clinical findings, however, differ completely.
Middle ear and mastoid
Introduction
The most frequent benign tumor in the middle ear and mastoid is the paraganglioma or glomus tympanicum tumor, followed by facial nerve schwannomas and congenital cholesteatomas. Malignant tumoral lesions of the middle ear are rare. The most frequent subtypes are middle ear adenocarcinoma and squamous cell carcinoma.
Attention should be paid to tumoral lesions in children and, more specifically, to rhabdomyosarcoma and Langerhans’ cell histiocytosis.
Paragangliomas
Paragangliomas arise from the extra-adrenal neural crest-derived paraganglia, the so-called “glomus bodies.” These glomus bodies lie along the nerves in the inferior temporal bone along the cochlear promontory in the middle ear, giving rise to the glomus tympanicum. They are also situated in the jugular foramen (glomus jugulare), in the common carotid artery bifurcation (glomus caroticum), and along the vagal nerve (glomus vagale). When involving the jugular foramen and the middle ear, they are called glomus jugulotympanicum tumors. Most of these jugulotympanic tumors arise in association with the glomus formations of the inferior tympanic branch of the glossopharyngeal nerve (Jacobson’s nerve) or the mastoid branch of the vagus nerve (Arnold’s nerve).
Paragangliomas account for 0.6% of all neoplasms in the head and neck region. Most (80%) are situated in the carotid bodies in the common carotid artery bifurcation and in the jugular foramen.
The glomus tympanicum tumor is the most common neoplasm of the middle ear. The peak age of incidence is during the fifth and sixth decades of life, and there is a clear female predisposition (3:1 ratio).
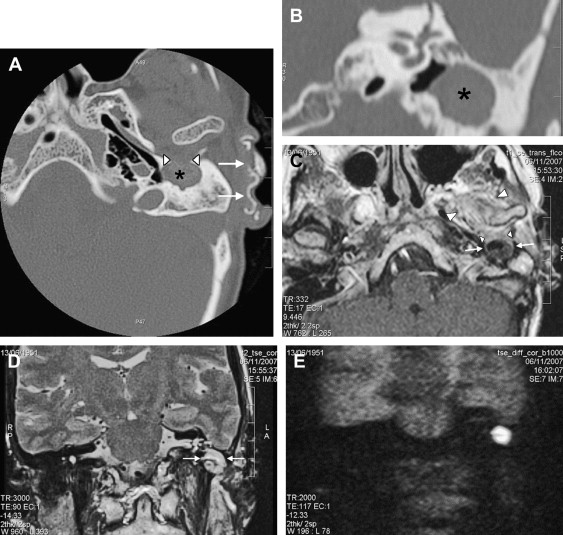
Pulsatile tinnitus is the most important and most common clinical symptom. In case of a large bulky tumor in the middle ear, conductive hearing loss can be found. Sensorineural hearing loss and vertigo can be caused by inner ear involvement. Numerous cranial nerves may be compromised because of involvement of the jugular foramen (ninth, 10th, and 11th cranial nerves) and the hypoglossal canal (12th cranial nerve). Clinically, the ear, nose, and throat surgeon finds a blue mass lesion behind the tympanic membrane. On a clinical basis, it is impossible to differentiate between a glomus tympanicum tumor, a glomus jugulotympanicum tumor, and vascular variants (eg, high-riding dehiscent jugular bulb, aberrant internal carotid artery). Because a surgical intervention or biopsy can potentially be harmful, it is essential that the radiologist knows the anatomic vascular variants and the cross-sectional imaging signs of glomus tympanicum and jugulotympanicum tumors.
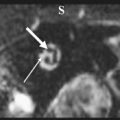
The glomus tympanicum tumor ( Fig. 7 ) presents on high-resolution CT (HRCT) as a nodular mass lesion situated anteriorly in the hypotympanum against the so-called “promontory.” The promontory is a bony bulge caused by the cochlea on the medial and anterior hypotympanic walls. The mass lesion can extend into the middle ear cavity, abutting the tympanic membrane. The ossicles are generally spared. If the mass is sufficiently large to fill the epitympanum, attic, and antrum, retention of fluid in the mastoid can be found. Bone erosion is usually not present. Although pulsatile tinnitus is a frequent clinical sign, glomus tympanicum tumors often cause conductive hearing loss. T1-weighted images show a strongly enhancing mass lesion located anteriorly in the signal void of the temporal bone pyramid. Signal intensities on T2-weighted images may vary but are predominantly intermediate.