Prostate surface membrane antigen (PSMA)-PET imaging has significantly shaped the clinical management of prostate cancer, from localized to metastatic disease. It outperforms conventional imaging in both primary staging and detecting recurrence. PSMA-PET incorporation into the clinical workflow can alter treatment decisions, though the impact of observed stage migration on patient outcomes has yet to be well-characterized. There is growing interest in using PSMA-PET to predict treatment response across all stages of prostate cancer, and to select patients for PSMA radioligand therapy. Use of PSMA-PET will continue to expand for clinical applications as its role becomes better defined through prospective studies.
Key points
- •
Prostate specific membrane antigen (PSMA)-PET has superior sensitivity and accuracy versus conventional imaging in detecting nodal and metastatic disease, which has significantly impacted clinical decision-making.
- •
PSMA-PET can more accurately reflect the total disease burden in metastatic castration-sensitive prostate cancer.
- •
PSMA-PET is increasingly being explored as a tool to predict and monitor therapeutic response and to stratify patients for PSMA-targeted therapy.
- •
While PSMA-PET has shown promise in guiding treatments and predicting treatment response, the impact on clinical outcomes is not well established in most settings.
| aPERCIST | Adapted PET Response Criteria in Solid Tumors |
| AS | active surveillance |
| BCR | biochemical recurrence |
| cN0 | clinically node negative |
| EAU/EANM | European Association of Urology/European Association of Nuclear Medicine |
| FDA | Food and Drug Administration |
| GG | grade group |
| ISUP | International Society of Urologic Pathology |
| mCRPC | metastatic castration-resistant prostate cancer |
| mCSPC | metastatic castration-sensitive prostate cancer |
| MDT | metastasis-directed therapy |
| omCSPC | oligometastatic metastatic castration-sensitive prostate cancer |
| PCa | prostate cancer |
| PCWG3 | PCa Working Group Criteria 3 |
| PLND | pelvic lymph node dissection |
| PPP | PSMA PET Progression |
| PPV | biochemical recurrence |
| PROMISE | prostate cancer molecular imaging standardized evaluation |
| PSMA | prostate specific membrane antigen |
| PSAM-RADS | PSMA Reporting and Data System |
| RECIP | Response Evaluation Criteria in PSMA PET/CT |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| RLT | radioligand therapy |
| RP | radical prostatectomy |
| rPFS | radiographic PFS |
| RT | radiation therapy |
| SRT | salvage radiation |
| WPRT | whole pelvis radiation therapy |
Introduction
Prostate cancer (PCa) is the second common cause of cancer and fifth most common cause of mortality from cancer in men worldwide. Localized PCa typically has an excellent prognosis, but metastatic disease carries increased morbidity and mortality. The incidence of metastatic PCa has been increasing since 2010, likely in part due to increased sensitivity of modern molecular-based imaging. The recognition and understanding of this stage migration is, therefore, paramount in determining the appropriate treatment course.
Prostate specific membrane antigen (PSMA) is a transmembrane glycoprotein that is primarily overexpressed in PCa epithelial cells. PSMA-guided PET imaging takes advantage of this by using a PSMA-ligand conjugated to a radiotracer. Targeted molecular imaging with PSMA-PET has transformed the diagnostic evaluation and staging of PCa in recent years. The US Food and Drug Administration (FDA) approved 68 Ga-PSMA-11 first in 2020 following prospective clinical trials showing PSMA-PET outperformed conventional imaging tools for initial staging and detecting recurrence of PCa. In addition to diagnostic improvements, there is growing interest in using PSMA-PET to predict and monitor for therapeutic response, further expanding its clinical utility.
This review aims to summarize the clinical application of PSMA-PET in diagnostic staging and prediction of treatment response across the spectrum of PCa from localized to metastatic disease.
Localized prostate cancer
Currently, European and the United States consensus guidelines incorporate PSMA-PET imaging into recommendations for initial staging of intermediate-risk and high-risk localized PCa. This inclusion was based on a growing body of evidence showing improved accuracy for detecting metastatic disease as part of staging compared with conventional imaging tools (ie, computed tomography [CT], magnetic resonance imaging [MRI], and nuclear medicine bone scan; Table 1 ). , , The detection of nodal and distant metastases can significantly impact recommendations for surgery (ie, radical prostatectomy [RP] and elective pelvic lymph node dissection [PLND]), volume of radiation therapy (ie, inclusion of elective nodal regions vs metastasis-directed therapy), and selection/duration of systemic therapy.
| Modality | Key Prospective Trials or Meta-Analysis | Sensitivity | Detection Rate | Specificity | Positive Predictive Value |
|---|---|---|---|---|---|
| Initial Staging | |||||
| Conventional (CT, MR imaging, bone scan) | Hovels et al, 2008 | 0.39–0.42 | NA | 0.82 | |
| 68Ga-PSMA-11 PET | NTR6830, PSMA-PreRP , | 0.4–0.42 | NA | 0.75–0.91 | 0.75–0.77 |
| 18F-DCFPyL PET | OSPREY Arm A, SALT , | 0.4–0.41 | NA | 0.94–0.98 | 0.54–0.87 |
| 18F-rhPSMA-7.3 PET | LIGHTHOUSE | 0.24 | NA | 0.96 | 0.65 |
| Biochemical Recurrence | |||||
| Choline C-11 PET | Evangelista et al, 2013, Fanti et al, 2016 | 0.86–0.89 | 0.62 | 0.89–0.93 | |
| Axumin (18F-Fluciclovine) PET | Wang et al, 2021 | 0.8 | 0.74 | 0.66 | |
| 68Ga-PSMA-11 PET | PSMA-BCR | 0.9 | 0.75 | 0.84 | |
| 18F-DCFPyL PET | CONDOR, OSPREY Arm B , | 0.96 | 0.59–0.66 | 0.71–0.82 | |
| 18F-rhPSMA-7.3 PET | SPOTLIGHT | 0.83 | 0.82 | ||
Low-Risk and Favorable Intermediate-Risk Prostate Cancer
Currently, PSMA-PET imaging is not included in consensus recommendations for staging of low risk and favorable intermediate-risk PCa. , However, there are potential roles for PSMA-PET for these patients that are under investigation. A pivotal decision point for patients with low-risk or favorable intermediate-risk PCa is whether to pursue treatment or active surveillance (AS). In general, PSMA-PET maximum standardized uptake value (SUVmax) appears to associate strongly with International Society of Urologic Pathology (ISUP) grade group (GG). For patients eligible for AS, SUVmax and adverse features on PSMA-PET (eg, MR imaging occult lesions, evidence of extraprostatic extension, or seminal vesicle invasion) have been shown to predict for upstaging on additional targeted biopsy or final histology after RP. , , A prospective study of 141 patients by Heetman and colleagues found that the number needed to scan for GG upstaging was 11 (based on targeted biopsy of PSMA-PET avid lesions with SUVmax ≥4). The optimal SUVmax cutoff remains unknown, however, as Roberts and colleagues showed that SUVmax ≥8 predicted GG ≥3 with a hazard ratio (HR) of 5.48 and associated with worse progression-free survival.
Integrating PSMA-PET SUVmax and multiparametric MR imaging (mpMRI) may provide additional guidance for patients eligible for AS. , , A prospective trial of 296 men that enrolled biopsy-naïve patients reported that the sensitivity for detecting GG 2+ disease was improved from 83% to 97% when combining PSMA-PET (using SUVmax ≥4) with mpMRI. The findings have been adopted into version 2 of the prostate cancer molecular imaging standardized evaluation (PROMISE) framework for PCa staging. More broad incorporation of PSMA-PET into clinical workflows for patients eligible for AS will require additional prospective studies and validated nomograms. One such study is the CONFIRM trial, which will evaluate the proportion of men with newly diagnosed PCa no longer deemed appropriate for AS following PSMA-PET (in addition to mpMRI and biopsy). Overall, current use of PSMA-PET in this setting should be done with caution, as cost and insurance coverage remain important barriers for many patients.
Unfavorable Intermediate-Risk and High-Risk Prostate Cancer
The landmark trials that led to FDA approval of PSMA-PET for staging of newly diagnosed PCa included patients with unfavorable intermediate-risk and high-risk disease. , , , 68 Ga-PSMA-11 was approved following 2 large multicenter trials. The phase 3 proPSMA study randomized 302 newly diagnosed men with high-risk features (at least one of prostate-specific antigen [PSA] ≥20 ng/mL, GG ≥3, or clinical stage ≥T3) to conventional imaging or PSMA PET-CT. Using a predefined reference standard (based primarily on confirmatory imaging and histopathology), the study showed that PSMA-PET outperformed conventional imaging in detecting nodal and distant metastases with greater sensitivity (85% vs 38%) and specificity (98% vs 91%). PSMA-PreRP was a single-arm prospective study of 764 patients, of whom 277 underwent RP and PLND to establish a standard of reference. 68 Ga-PSMA-11 PET detected pelvic lymph node metastases with 40% sensitivity and 96% specificity.
Large prospective studies using 18 F-DCFPyL have shown similar performances to 68 Ga-PSMA-11 PET for detecting pelvic lymph node metastases. , OSPREY was a phase 2/3 multicenter study with 2 cohorts: Cohort A enrolled patients with high-risk PCa intended for RP + PLND and Cohort B enrolled patients with suspected recurrence. Cohort A reported 40% sensitivity and 98% specificity, with higher positive predictive value compared with conventional imaging (87% vs 28%). The multicenter SALT trial also showed similar results, reporting 41% sensitivity and 94% specificity. The SALT trial, which used a ≥8% risk probability for LN metastasis for performing elective PLND, noted that the median size of nodes not detected on PSMA-PET was 1.5 mm, which led the authors to conclude that PSMA-PET may be inadequate to replace elective PLND for staging due to the presence of small metastatic deposits. More recently, the FDA approved 18 F-rhPSMA-7.3 for staging of newly diagnosed PCa based on the prospective LIGHTHOUSE study. While the reported specificity for detecting pelvic lymph node metastases was 93%, sensitivity ranged from 23% to 30% among 3 readers, which is lower than for other approved radioligands.
Results of PSMA-PET staging can significantly impact treatment decisions. For patients deemed appropriate for definitive radiation therapy (RT), treatment fields and boost volumes can vary based on the extent of disease. For high-risk clinically node negative (cN0) disease defined on conventional imaging, RTOG 9413 showed that elective whole pelvis RT (WPRT) improved progression free survival (PFS) compared with prostate-only RT when given with neoadjuvant hormone therapy. However, it is unclear whether cN0 disease staged by PSMA-PET is sufficient to omit WPRT, even though PSMA-PET has a high negative predictive value ranging from 81% to 90%. , , In fact, the randomized prostate-only or whole-pelvic radiation therapy in high-risk prostate cancer (POP-RT) trial showed improved biochemical failure-free survival with WPRT compared with prostate-only RT (when combined with 2 years of androgen deprivation therapy [ADT]), despite 80% of patients being cN0 based on PSMA-PET. This seems to suggest the importance of other risk factors in predicting imaging-occult pelvic disease for high-risk patients, and support findings from the SALT trial regarding limitations of PSMA-PET in the detection of small metastases. More frequently in the clinic, PSMA-PET findings lead to more comprehensive treatment volumes. A post hoc analysis of 73 patients treated on trial found that PSMA-PET positive nodes altered 32% of RT plans due to initial under-coverage of the clinical target volume.
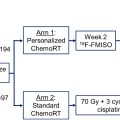
While the use of PSMA-PET exposes issues with target coverage based on conventional imaging, the long-term impact of defining RT volumes and selecting type and duration of hormone therapy based on PSMA-PET has yet to be determined in a prospective randomized setting. While it may seem appropriate to determine therapy based solely upon this more sensitive imaging modality, there is currently a lack of evidence to support this approach. Therefore, guidelines continue to support offering definitive therapy for patients with cN0 or cM0 disease defined based on conventional imaging regardless of extraprostatic PSMA disease. The multi-institutional phase 3 trial PSMA-dRT intended to evaluate the impact of PSMA-PET on treatment outcomes, but closed early due to enrollment. A 2-year update with 54 patients randomized showed upstaging in 17% of patients with PSMA-PET, but no difference in PFS. Two phase 3 trials are currently enrolling: PATRON, which will randomize to PSMA-PET ( 18 F-DCFPyL or 18 F-PSMA-1007) versus conventional imaging ; and EMPIRE-II, which will randomize to PSMA-PET ( 68 Ga-PSMA-11) versus fluciclovine-PET, following results of EMPIRE-I, which showed improved 3-year event-free survival using fluciclovine-PET over conventional imaging.
For patients deciding between RP with PLND and RT, PSMA-PET may assist with shared decision making. PSMA-PET improves existing predictive nomograms for predicting pelvic lymph node metastases, and PSMA-PET N1/M1 status is prognostic for biochemical recurrence (BCR) after surgery. Importantly, prediction of pathologic N1 status may alter the decision to take a surgical approach, given the increased toxicity associated with adjuvant RT and the potential clinical determinant in delaying RT among pathologic node positive patients. ,
The role of follow-up PSMA-PET after definitive treatment of localized PCa is poorly defined. Existing data on PSMA-PET response and clinical outcomes are from retrospective work. Chen and colleagues conducted a multivariable analysis of PSMA-PET response in 75 high-risk patients treated with ADT plus docetaxel or abiraterone followed by definitive RT. They found that post-treatment SUVmax less than 8.5 was an independent predictor of biochemical PFS. However, change in SUVmax appears to depend on treatment type, making these findings difficult to generalize. For example, Onal and colleagues showed in an analysis of 71 intermediate-risk PCa patients that PSMA-PET complete metabolic responses occurred in 40% of patients receiving ADT + RT compared with 0% in RT alone. Further, the optimal timing of post-treatment PSMA-PET remains unknown, adding additional uncertainty to its application in identifying treatment response. Nonetheless, given that PSA and PSMA response do not appear to be highly concordant, there may be a role for PSMA-PET in routine follow-up once the relationship between post-treatment imaging changes and outcomes are better characterized.
Recurrent prostate cancer
Consensus guidelines recommend use of PSMA-PET in the detection of suspected PCa recurrence. Prospective studies have demonstrated high sensitivity and accuracy in detecting and locating BCR using 68 Ga-PSMA-11, , 18 F-DCFPyL, , and 18 F-rhPSMA-7.3 (see Table 1 ). The PSMA-BCR study (median PSA 2.1 ng/mL) reported that PSMA-PET using 68 Ga-PSMA-11 yielded a positive predictive value (PPV) of 84% based on histopathology follow-up and 92% using a composite metric combining histopathology, imaging, and post-treatment PSA. Within the entire cohort, PSMA-PET was able to localize recurrent disease in 75% of patients with the detection rates improving with higher PSA levels. The association of detection rate with PSA levels was recapitulated in a separate multicenter prospective study by Abghari-Gerst and colleagues, which enrolled patients with BCR after RP (PSA >0.2 ng/mL > 6 weeks post-op), definitive radiation therapy (≥2 ng/mL from PSA nadir, per Phoenix definition), or postoperative RT. With histopathology as reference, the overall PPV was 82%, and SUVmax was significantly higher for true positive lesions.
The performance of 18 F-DCFPyL was shown to be similarly high, with a correct localization rate of at least 84.8% and detection rate of at least 59% in the CONDOR study (median PSA 0.8 ng/mL). PSMA-PET-based detection and localization led to change in management of 64% of individuals. Interestingly, changes in management included both shift from salvage local therapy to systemic therapy and vice versa, underscoring the importance of accurately characterizing both volume and location of recurrence for escalating or de-escalating therapy. Cohort B of the OSPREY study (median PSA 7.1 ng/mL), which studied the ability of 18 F-DCFPyL PSMA-PET to detect nodal and distant metastases, reported a sensitivity of 96% and PPV of 82%. Notably, PSMA-PET detected metastatic disease in 58% of patients with BCR who otherwise had no discernible lesions on conventional imaging.
Recently, the FDA approved 18 F-rhPSMA-7.3 among patients with BCR following results of the SPOTLIGHT trial. The detection rate was 51% to 54% with both histopathology and confirmatory imaging as reference and higher at 81% using histopathology alone, which is comparable to other radioligands. The histopathology-based patient level PPV was comparable as well at 82%.
Similar to the impact in localized prostate cancer, PSMA-PET can significantly alter management in the recurrent setting. This was shown in the prospective randomized phase 3 PSMA-SRT trial, which randomized patients with BCR to salvage radiation (SRT) or 68 Ga-PSMA-11 PET prior to RT planning. Notably, 43% of the control arm had fluciclovine-PET. Considering the use of advanced imaging in the control arm, the PSMA-PET arm still showed a 23% absolute difference in major changes in management. Overall, 33% of patients in the PSMA-PET arm had treatment changes related to PSMA-PET findings. However, the impact of these changes on the primary endpoint of BCR-free survival has not been reported yet.
Metastatic castration-sensitive prostate cancer
Within metastatic castration-sensitive prostate cancer (mCSPC), disease volume plays an integral role guiding treatment recommendations including selection of hormone therapy, , docetaxel, , prostate-directed therapy, , and metastasis-directed therapy. Disease volume has commonly been stratified into low-volume/oligometastatic and high-volume/polymetastatic. However, these definitions have been mostly commonly evaluated in the setting of conventional imaging. Imaging trials using PSMA-PET have shown improved sensitivity to detect metastatic disease, , thereby upstaging patients from BCR to metastatic, and low-volume to high-volume. This may have a profound impact on both prognosis and treatment options. Unfortunately, there is currently limited evidence to how this stage migration should be considered in the context of clinical trials from the era of conventional imaging.
Metastasis-Directed Therapy
Oligometastatic mCSPC (omCSPC) is a state of limited volume disease often defined as ≤3 to 5 metastases, which appears to derive benefit from metastasis-directed therapy (MDT). This was first identified within the STOMP trial which randomized 62 patients with ≤3 metastases on 11-choline PET to observation versus MDT. This trial demonstrated MDT improved ADT-free survival from 13 to 21 months. The ORIOLE trial similarly randomized patients with ≤3 metastases to observation vs MDT, but used conventional imaging for staging. Importantly, patients on the ORIOLE trial underwent PSMA-PET imaging at diagnosis, but the investigators were blinded to the results. While the ORIOLE trial similarly demonstrated a benefit of MDT (6-month PFS 81% vs 39%), patients treated with MDT who had untreated conventionally occult, PSMA-PET avid lesions demonstrated significantly worse PFS (HR = 0.26, P =.006) than those with total PSMA-PET consolidation. These findings suggest the importance of highly sensitive staging imaging when identifying patients for MDT to ensure total disease consolidation can be achieved.
While both choline-PET and PSMA-PET offer improved sensitivity over conventional imaging, PSMA-PET appears superior for guiding total consolidative MDT. Mazzola and colleagues demonstrated that PSMA-PET-directed MDT was associated with improved 1-year ADT-free survival (89.6% vs 73.2%, P =.01) as compared with choline-PET-directed therapy. Deijen and colleagues similarly demonstrated PSMA-PET-directed MDT was associated with significantly longer median ADT-free survival (34.0 vs 14.7 months, P =.01) compared with choline-PET-directed MDT. This improvement is likely due to the increased detection of occult metastatic disease with PSMA-PET and consistent with the results of the ORIOLE trial.
In addition to increasing metastatic detection for total disease consolidation, PSMA-PET also appears to provide prognostic information. Sutera and colleagues reviewed 295 patients with metachronous omCSPC detected either on molecular (PSMA-PET, choline-PET, or fluciclovine-PET) imaging only or conventional imaging. Patients with molecular-only omCSPC were found to have better overall survival (OS) both from time of metastasis (HR = 0.13, P =.007) as well as from initial localized disease (HR = 0.12, P =.006). Further, molecular-only omCSPC was associated with fewer TP53 and more SPOP somatic mutations, indicating a less aggressive disease biology. Taken together, the results suggest that molecular-only omCSPC represents a more indolent disease state than those with metastases detectable on conventional imaging, rather than simply identifying patients earlier in their disease course. This difference in disease biology has potential to be leveraged for improved therapeutic precision in these 2 groups of omCSPC.
PSMA-PET not only holds promise for proper patient selection and prognostication within patients with omCSPC, but it may also be used as an indicator for treatment response to MDT. Greco and colleagues reported a single-institution phase II study evaluating PET response following MDT in oligometastatic disease across multiple histologies. This study included 147 patients with PCa (who underwent 68 Ga-PSMA PET) and demonstrated change in SUVmax was associated with locoregional failure. More recently, an international multi-institutional review of men with omCSPC with pre-MDT and post-MDT PSMA-PET was reported. This work identified 131 patients with 261 treated metastases who underwent PSMA-PET approximately 6 months following MDT. Patients were classified as being PSMA responders (all lesions with at least 30% reduction in SUVmax) or PSMA nonresponders (at least 1 lesion with <30% reduction in SUVmax). Following stereotactic ablative radiation therapy (SABR), 70.2% of patients were classified as PSMA responders, who were found to have significantly improved median metastasis-free survival (MFS) of 39.9 versus 12 months ( P =.001) compared with PSMA nonresponders. Follow-up work with this cohort utilized machine learning incorporating both radiomic (pretreatment and post-treatment PSMA-PET) and clinical features was able to predict 2-year MFS with an accuracy of 75% to 80%.
Prostate-Directed Therapy
As previously mentioned, disease volume is also used to determine the role of prostate-directed therapy. The STAMPEDE arm H trial demonstrated an overall survival benefit with radiotherapy to the prostate for patients with low-volume but not high-volume de novo metastatic disease. Importantly, this definition was disease volume was based on conventional imaging. More recently, the PEACE-1, a 2×2 factorial design randomized trial, included radiotherapy to the prostate for patients with low-volume disease. This trial, however, similarly used a conventional imaging definition of disease volume. It, therefore, remains unclear if there is a differential benefit to prostate-directed therapy for patients with conventionally detected low-volume disease with PSMA-PET detected low-volume versus high-volume disease.
Metastatic castration-resistant prostate cancer
Several recent studies have explored the utility of PSMA-PET in predicting treatment response, disease progression, and overall survival in metastatic castration-resistant prostate cancer (mCRPC) patients, especially in the setting of patients undergoing radioligand therapy (RLT) with 177 Lu-PSMA-617. 177 Lu-PSMA-617 has been shown to prolong radiographic PFS and OS, while also improving quality of life compared with the standard of care in patients with mCRPC. , However, a subset of patients remains resistant to this treatment, with resistance observed in 30% of patients in the VISION trial and 17% in the TheraP trial. , Identifying those who will benefit most from PSMA-RLT is crucial for tailoring therapeutic options for mCRPC patients.
Predictive Value of Prostate Specific Membrane Antigen-PET and Patient Selection for Radioligand Therapy
Biomarkers based on PSMA-PET have been investigated in multiple retrospective and prospective studies involving RLT, including VISION, TheraP, LuPSMA. In order to develop a nomogram for predicting outcomes after PSMA-RLT, Gafita and colleagues conducted a multicenter retrospective study of 270 mCRPC patients in which they identified 6 variables as independent predictors of OS. Notably, the SUVmean of whole-body tumor burden, the number of lesions, and the presence of liver metastases remained significant on multivariable analysis. TheraP, a multicenter randomized phase 2 trial randomizing mCRPC patients to 177 Lu-PSMA-617 versus cabazitaxel, reported whole-body tumor PSMA-PET SUVmean ≥10 was associated with greater PSA response and radiographic PFS (rPFS) for PSMA-RLT compared with cabazitaxel. , Although the cutoff of SUVmean ≥10 did not translate into a difference in OS within the 177 Lu-PSMA-617 treatment arm in the updated analysis, SUVmean ≥10 was still prognostic for OS (HR: 0.58, 95% confidence interval [CI]: 0.40–0.83, P =.0033) after adjusting for randomized treatment. A secondary analysis of the VISION trial also showed that whole-body tumor SUVmean was the strongest imaging predictor of rPFS and OS after PSMA-RLT. These findings provide guidance for the optimal use of 177 Lu-PSMA-617 in mCRPC patients as an alternative to cabazitaxel.
The presence of PSMA-negative lesions has also emerged as an important variable for predicting patient outcomes and for determining eligibility for PSMA-RLT. According to the phase III VISION trial, patients must have had at least one PSMA-positive metastatic lesion and no PSMA-negative lesions to qualify for treatment. Patients who did not meet the VISION inclusion criteria-either due to low PSMA expression or the presence of PSMA-negative lesions (defined as SUVmax less than or equal to liver background), had poor outcomes following PSMA-RLT. , Furthermore, discordance between fluorodeoxyglucose-PET (FDG-PET) and PSMA-PET uptake also portends worse outcomes. In a prospective study by Telli and colleagues, both 18 F-FDG and 68 Ga-PSMA PET/CT scans were used to comprehensively assess tumor burden and heterogeneity in 52 mCRPC patients who received 2 to 6 cycles of PSMA-RLT. The study found that discordant imaging findings, where FDG uptake exceeded PSMA uptake in the majority of lesions (defined as FDG > PSMA disease), were associated with poorer OS and shorter PFS. Similarly, discordant findings on bone scintigraphy, where 99m Tc-HDP uptake was higher than PSMA uptake in most bony lesions (defined as bone scan > PSMA disease), were linked to shorter PFS. Michalski and colleagues further corroborated these findings, reporting significantly lower median OS in patients with at least one FDG+/PSMA-lesion at baseline PET/CT (6.0 ± 0.5 months, 95% CI: 5.0–7.0 months) compared with those without any FDG+/PSMA-lesions (16.0 ± 2.5 months, 95% CI: 11.2–20.8 months).
Although clinical trials cannot be directly compared, both the LuPSMA and TheraP trials excluded patients with discordant PET lesions (ie, FDG positive with no or low PSMA avidity) and resulted in PSA response of 64% and 66%, respectively. , This contrasts with the VISION trial, where PET-only screening resulted in a lower PSA response rate (46%). The advantages of using dual-tracer PET screening to select patients for PSMA-RLT warrant further investigation. Efforts are ongoing to develop dual-tracer criteria for PSMA-RLT eligibility and for predicting outcomes. Adnan and Basu have proposed a novel 6-tier integrated dual-tracer PET/CT scoring system, the “Pro-PET” score, which combines 68 Ga-PSMA and FDG PET imaging to predict therapeutic outcomes and prognosis in mCRPC. There are ongoing studies to evaluate dual-tracer criteria prospectively, including the Triple-Tracer strategy against Metastatic PrOstate cancer (3TMPO) study.
Finally, the presence of liver metastases in mCRPC is associated with a poorer response to many forms of systemic therapy, likely due to the differentiated tumor microenvironment and intertumoral heterogeneity. Though this does not affect patient selection for PSMA-RLT, combination approaches or augmented PSMA-RLT may be considered to maximize the antitumor activity of RLT in the liver.
Assessment of Treatment Response
The evaluation of treatment response in mCRPC typically follows the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 or the PCa Working Group Criteria 3 (PCWG3), using conventional imaging modalities such as CT and bone scans. , However, changes in PSMA expression detected on PSMA PET/CT scans after treatment offer a more sensitive and specific indicator of treatment response, surpassing traditional imaging in its ability to specifically target prostate cells. Despite its advantages, the role of PSMA PET/CT in routine clinical practice for response assessment remains less well-defined. Nevertheless, PSMA PET/CT progression criteria have been established for clinical trials, providing a standardized framework for evaluating treatment response in mCRPC patients. Two commonly used PSMA PET/CT reporting frameworks include the PSMA Reporting and Data System (PSMA-RADS) and the PROMISE framework. PSMA-RADS employs a 1 to 5 scale to assess the likelihood that tracer accumulations represent malignancy or benign processes. In contrast, the PROMISE framework offers a 3-tiered assessment, characterizing lesions, describing the metastatic extent, and quantifying tumor volume (PSMA-VOL) within molecular imaging regions, while considering organ-specific biological factors and their differential impact on patient outcomes. Several response assessment systems tailored to PCa and PSMA PET/CT imaging have been developed to evaluate treatment response in mCRPC. The Adapted PET Response Criteria in Solid Tumors (aPERCIST) is a standardized method specifically designed for assessing treatment response in PCa using PET imaging, typically with PSMA PET/CT.
Additionally, response assessment frameworks can be built on the PROMISE report. Two notable examples are the Response Evaluation Criteria in PSMA PET/CT (RECIP) and the PSMA PET Progression (PPP) criteria. RECIP evaluates changes in PSMA volume and the emergence of new lesions, making it particularly suitable for advanced mCRPC, while PPP focuses on individual lesions, making it more applicable to limited disease cases. A retrospective study by Shagera and colleagues specifically examined the potential use of PSMA-PET as an imaging biomarker for assessing and predicting outcomes in mCRPC patients treated with androgen receptor pathway inhibitors. The study compared 2 proposed PSMA response criteria—the European Association of Urology/European Association of Nuclear Medicine (EAU/EANM) criteria and RECIP 1.0. Both criteria demonstrated that PSMA responders had significantly longer median overall survival compared with nonresponders (54 vs 22 months). Although PSMA PET/CT shows promise as a response assessment tool in both clinical trials and routine practice, further prospective studies are necessary for validation.
Summary
PSMA-PET has been a disruptive imaging technology that has transformed the diagnostic evaluation and staging of PCa across its entire spectrum, from localized to metastatic disease. Its superior sensitivity and accuracy in detecting nodal and metastatic disease compared with conventional imaging methods have led to its widespread adoption in clinical practice. This integration has significantly influenced clinical decision-making, including the extent of surgery or radiation therapy and the selection of systemic treatments. PSMA-PET is crucial in detecting metastatic disease and predicting treatment response, especially in the context of MDT for omCSPC. Additionally, PSMA-PET’s ability to stratify patients based on disease volume and biological characteristics enables more precise and individualized treatment approaches and plays a critical role in selecting patients with mCRPC for PSMA-targeted therapy. It is also becoming essential in monitoring treatment response in mCRPC, particularly after PSMA-RLT or systemic therapy, where it can predict therapeutic outcomes and overall survival. However, concerns remain about the sensitivity of PSMA-PET in detecting small metastatic deposits, which may limit its ability to replace existing procedures such as elective PLND. While PSMA-PET has shown great promise, further research is needed to validate its use in various clinical settings. Presently, many of the randomized trials that guide treatment decisions in prostate cancer have been based upon conventional imaging and it remains unclear if these treatments hold the same benefit for patients with conventionally occult, PSMA-PET avid disease. Although further prospective studies are needed, the advent of this highly sensitive and specific imaging has led to a new era of prostate cancer disease management.
Clinics care points
- •
PSMA-PET significantly outperforms conventional imaging (CT, MRI, bone scans) for detecting nodal and distant metastases in intermediate- and high-risk prostate cancer.
- •
PSMA-PET leads to upstaging through detecting occult metastatic disease that may alter the need for systemic therapy, radiation fields, or surgical approaches, though prospective data is limited.
- •
PSMA-PET has high accuracy in detecting recurrent prostate cancer at low PSA levels, identifying disease not visible on conventional imaging, which may alter salvage treatment strategies.
- •
Within oligometastatic prostate cancer, PSMA-PET more fully captures the extent of metastatic disease allowing for more accurate total consolidation with local therapy.
- •
PSMA-PET is critical for selecting patients with metastatic castration-resistant prostate cancer for PSMA-targeted radioligand therapy (177Lu-PSMA-617), as high PSMA expression correlates with better treatment responses, while PSMA-negative lesions predict worse response to therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree