Interpreting the Mammogram
By far the most important function of mammography is the earlier detection of breast cancer in an effort to decrease the death rate from these malignancies. The performance and acquisition of high-quality mammography is the key to the ability to accomplish this task. No amount of interpretive expertise can correct for the fact that the lesion is not imaged because of poor positioning, is hidden by underpenetrated tissue, or is overlooked because the image is blurred. Similarly, no observer is perfect, and all observers are subject to periodic failure to perceive a lesion that is visible in retrospect. Although not the standard of care, double reading should be encouraged, but it must be accomplished efficiently so that there is no significant increase in cost. Double reading reduces the false-negative rate, but since it is not reimbursed, it should not be considered the standard of care. If double reading were required, the cost of screening would be prohibitively expensive, and screening would cease to be available. If women are denied access to screening because of increased cost, then clearly there can be no benefit from screening, and requiring double reading would be detrimental to women’s health. Nevertheless, a systematic approach to the entire screening process can improve efficiency, maintain quality, and help to lead to the earlier diagnosis of breast cancers.
Batch Reading Versus Immediate Interpretation
In the 1980s, the media exposed some facilities and some radiologists who were providing poor-quality mammography and were failing to inform women who were found to have a cancer that they had a problem. As a result, the diagnosis of their cancers was delayed. These stories led to a congressional investigation that ultimately resulted in the passage of the Mammography Quality Standards Act (MQSA). The MQSA was a mandate to produce basic standards for mammography, including equipment; qualifications for the radiologists, technologists, and physicists involved; quality control; and quality assurance programs. The Food and Drug Administration (FDA) was assigned to oversee a national program to try to insure that mammography facilities adhered to basic quality standards.
In an effort to reduce the chance that a significant finding would not be brought to the patient’s attention, causing her diagnosis to be delayed, the media exposés urged women to obtain their mammography reports before they left the mammography screening facility. Other facilities used the immediate interpretation of mammograms as a marketing tool, and many women began to expect that their screening study should be interpreted while they waited. It is now apparent that this is a very expensive and a somewhat risky practice. We found that when trying to provide immediate interpretations, patients began to “back up” in the waiting room. Some who were in a rush to leave would pressure the technologists for the reading, and the technologists would pressure the radiologists. This forced the radiologist to rush the interpretation, raising the risk that a lesion would be overlooked. We had performed a study to evaluate the benefit of double reading. The study included over 6,000 women among whom we detected 39 cancers. Our first reader read slowly and methodically and took well over an hour to review 40 mammograms. The mammograms were then read again by a second reader, who reviewed all the studies in a few minutes. The main reader found 36 of the cancers. The “quick” (second) reader found 3 malignancies that had been missed by the main reader. However, the quick reader missed 8 cancers that were found by the main reader. Our study points out the fact that two readers are better than one, but if there is only one reader, that single reader should read slowly and methodically and not be rushed because a patient is waiting for the report. As a result of our study, we now provide a second reading at no charge to the patient, but we have stopped providing an immediate reading because of the danger in rushing the reading.
Providing immediate interpretations of screening mammograms also virtually precludes the practice of double reading. Since the latter is not reimbursed, the only way to provide a double read is to interpret studies efficiently so that the second reading takes a minimum amount of time. Efficient double reading is facilitated by having the mammograms interpreted in batches. For these reasons it is probably not a good idea to read screening mammograms while the patient waits. If mammograms are not interpreted “on line” and the patient is not provided with her report before leaving the facility, then, by law, there needs to be a system in place to be sure that the report is generated and sent to the patient within 30 days of the study. Although it can be debated as to whose responsibility it is to try to insure that a patient complies with follow-up recommendations, we believe it is a good idea to have a system that tries to monitor whether women comply with those recommendations. This too must be organized in an efficient manner since it can rapidly become very labor intensive and expensive, and this, too, is not covered by reimbursement.
Interpreting screening studies in batches can reduce the cost by maximizing the radiologist’s productivity. The disadvantage of the patient’s not having an immediate report is more than offset by the reduced expense and the added advantage that reading mammograms on an alternator permits double reading. As has been shown in the interpretation of chest x-rays (1), barium enemas, and other imaging studies, there is a psychovisual phenomenon that guarantees that all radiologists will periodically fail to perceive significant abnormalities. Mammographic interpretation is no exception. Every radiologist, no matter how skilled and no matter how hard he or she concentrates on looking for cancer, is guaranteed to periodically overlook cancers that are visible on mammograms. Because different readers overlook different findings, having more than one reader interpret a mammogram reduces the error rate. Interpreting mammograms on an alternator permits multiple reviewers with little increase in cost. In our own study, double reading in this fashion increased our breast cancer detection rate by 7%. Bird (2) found that a second reviewer increased the cancer detection rate by 5%, and Tabar et al (3) have reported as high as a 15% improvement in cancer detection with double reading. This increased yield from double reading has also been documented by Thurfjell et al (4). Double reading can be accomplished using digital mammography once the workstations allow random lists to be generated.
Detection, Diagnosis, and Diagnostic Accuracy
Mammography is invaluable in the detection of anomalies (benign and malignant lesions) in the breast (detection), but it is less suited for the differentiation of those anomalies into benign or malignant (diagnostic) categories. As breast imaging techniques have evolved, a better understanding of the underlying histopathologic changes that account for some of the mammographic findings has developed. Nevertheless, the problem remains that the diagnosis (in the true sense of the word) of many lesions, based on imaging criteria, often defies the high levels of certainty required if a tissue diagnosis is to be avoided. In view of the important consequences of early breast cancer detection and the high level of safety in obtaining a tissue diagnosis, the accuracy of a noninvasive diagnostic study that is used to avoid a biopsy should approach 100%.
Despite uncertainties, criteria have been established to assist in diagnosis. Before the instigation of an invasive procedure, it is important to analyze and categorize anomalies in the breast so that appropriate action can be taken. Mammography and appropriate diagnostic techniques, such as ultrasound for differentiating cysts from solids, should be used to maximize the diagnosis of early cancers and reduce the need to biopsy benign lesions whenever possible.
Image Review
Digital Mammography versus Film/Screen
Virtually all of the criteria that have been developed for the interpretation of film/screen mammograms apply to digital mammograms as well. These criteria also apply to the analysis of slices in digital breast tomosynthesis (DBT). One of the advantages of DBT is that much of the diagnostic requirements that are engendered by findings on 2D mammograms are no longer required when DBT is used. DBT eliminates overlapping structures, the margins of masses and the morphology of calcifications are more clearly seen, and the three-dimensional locations of findings are accurately determined on the screening study.
Use an Organized Approach
The acquisition and interpretation of any x-ray mammographic study should be undertaken in an organized and structured fashion. High-quality mammography is required, and the images should be viewed in an appropriate environment with reduced ambient light and good viewing conditions. Images should be reviewed using a consistent viewing scheme. The radiologist should systematically search the images for masses, calcifications, areas of asymmetry, architectural distortion, and changes from previous studies, when they are available. Interpretive decisions should be based on established thresholds for intervention, and the interpreter should have reproducible and consistent reasons for interpretive decisions.
View Box and Workstation Analysis
The View Box or Workstation
The viewing conditions for mammography are important. Although ideal viewing conditions have never been scientifically determined, there are general principles that apply. When reviewing modern, high-contrast films with a high
maximum film density, bright backlighting is needed. In general, the light output of a mammography view box should probably be twice as bright as a standard light box. Although there have never been any truly scientific studies of the appropriate light output, Haus et al (5) found that 5 experts used view boxes with approximately 3,500 nits (candela/m2) of light output to view high-contrast mammographic images. Workstations for digital mammography produce much less light. However, I do not believe that a workstation needs to produce nearly as much light as a view box. Because a film image consists of varying shades of gray that block the passage of light through the film, the view box must produce sufficiently strong light to “push” it through the film, which dramatically decreases the light as it passes through. A workstation only needs to produce enough light so that the radiologist can see the structures. Workstation illumination should not be compared to the maximum light output of a view box. A more valid comparison would be to measure the brightness and contrast of a white structure seen on film to its brightness and contrast as portrayed on a workstation. Modern workstations approved for mammography produce sufficient light for viewing digital mammograms.
maximum film density, bright backlighting is needed. In general, the light output of a mammography view box should probably be twice as bright as a standard light box. Although there have never been any truly scientific studies of the appropriate light output, Haus et al (5) found that 5 experts used view boxes with approximately 3,500 nits (candela/m2) of light output to view high-contrast mammographic images. Workstations for digital mammography produce much less light. However, I do not believe that a workstation needs to produce nearly as much light as a view box. Because a film image consists of varying shades of gray that block the passage of light through the film, the view box must produce sufficiently strong light to “push” it through the film, which dramatically decreases the light as it passes through. A workstation only needs to produce enough light so that the radiologist can see the structures. Workstation illumination should not be compared to the maximum light output of a view box. A more valid comparison would be to measure the brightness and contrast of a white structure seen on film to its brightness and contrast as portrayed on a workstation. Modern workstations approved for mammography produce sufficient light for viewing digital mammograms.
Regardless of whether the image is on film or a computer, because extraneous light reduces the eye’s ability to perceive low-contrast objects, the images should be masked to reduce the glare from around the images. Glare can dramatically interfere with visualization of details on the mammogram, and it should be eliminated as much as possible. Ambient light also compromises viewing, and reading rooms should have low light levels. The view boxes used by the technologists should be matched to those used by the radiologists, so that properly exposed mammograms appear the same, facilitating quality control. Fortunately, because of the ability to “window and level” digitally acquired mammograms, the technologist’s and radiologist’s workstations do not have to be as closely matched, but the technologist needs to be able to see on her workstation the same structures that the radiologist can see. This not only ensures that she is able to be certain of proper positioning, exposure, and sharpness of the image, but, when she is performing diagnostic studies, she must be able to see the area of concern so that she can obtain the needed images. The technologist workstation must have sufficient image quality so that she can see if there is any blur from motion.
Positioning Images for Viewing
Images should be positioned in a standard and consistent fashion on the view box, as well as on computer monitors. They should be placed the same way each time to minimize left-right confusion. Most reviewers find that it is preferable to match the mediolateral oblique (MLO) images from each breast and place them back-to-back as mirror images, and to position the craniocaudal (CC) views base-to-base as mirror images with the lateral (axillary) portions of both at the top. Although the convention is to place the mammograms as if the patient were facing the viewer, many reviewers of film/screen mammograms prefer to have the dull emulsion side up to minimize reflection from the nonemulsion surface. This places the left breast on the left and the right on the right. Most mammography workstations permit the radiologist to have the images displayed in any way that is preferred.
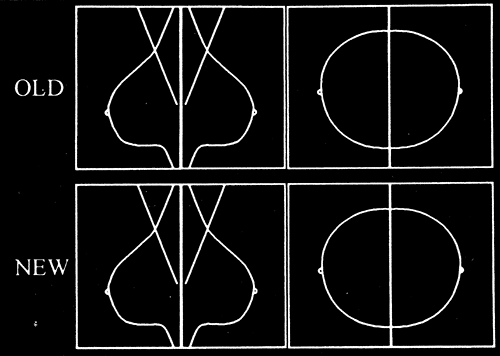
If there are two banks of view boxes, we place the new films on the bottom row with older films above (Fig. 13-1), although the specific arrangement of the films is up to the preference of the interpreter. Because subtle changes may not be apparent from 1 year to the next, we routinely compare films from 2 or more years earlier with the present study. There has been one study that suggested that the most recent previous study should be used (6), but the reasoning for this paper was flawed. It is rare that a significant change is not evident from 1 year to the next, but there are rare occasions where a change is much more apparent when even older studies are compared to the most recent (Fig. 13-2). A small study such as the one performed by Sumkin et al would not show these rare cases. Consequently, the use of 2-year-old or older studies is based on anecdote not science, but it is the prudent approach.
If digital mammograms are being reviewed, I believe that it is advantageous to display the present study on the same monitor as the previous study and to be able to rapidly toggle back and forth between the two studies. By shifting back and forth, new findings become apparent because the eye is very good at noticing a change between two pictures displayed in the same orientation. A new mass will be more apparent if the images are toggled rapidly in the same field of view than if the observer looks between two images on separate monitors.
Quality Control at the Viewing Station
Checking Breast Positioning
The images should first be evaluated with respect to quality. The breasts should ideally have been positioned symmetrically for the exposures so that the radiologist can better
appreciate any asymmetries. The technologist should have tried to maximize the amount of tissue projected onto the detector. The nipple should be in profile, if possible. This is not an absolute requirement: If, to position the breast to maximally visualize the breast tissues, the nipple goes out of profile, the visualization of the tissues takes precedence. If there is a question as to whether what is being seen is a subareolar mass or the nipple not in profile (which can look like a mass), an additional view of the subareolar tissues with the nipple in profile will clear up any confusion.
appreciate any asymmetries. The technologist should have tried to maximize the amount of tissue projected onto the detector. The nipple should be in profile, if possible. This is not an absolute requirement: If, to position the breast to maximally visualize the breast tissues, the nipple goes out of profile, the visualization of the tissues takes precedence. If there is a question as to whether what is being seen is a subareolar mass or the nipple not in profile (which can look like a mass), an additional view of the subareolar tissues with the nipple in profile will clear up any confusion.
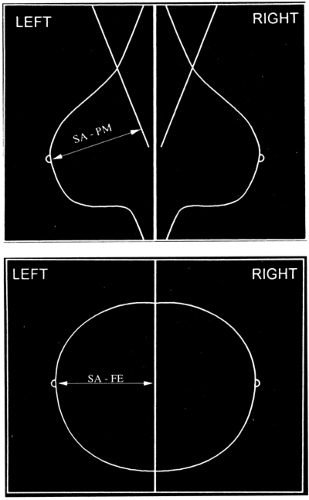
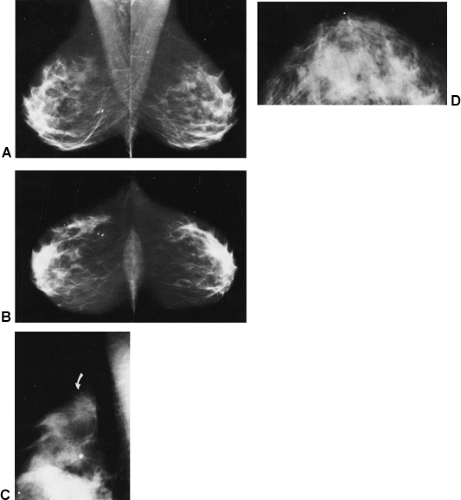
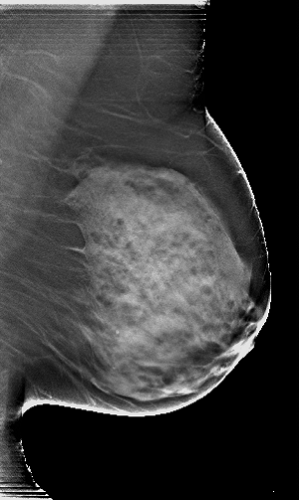
Eklund and Cardenosa (7) described measurements that provide some indication that the maximum tissue to be imaged is on the films. Body habitus and patient infirmities may preclude optimal positioning, but there are some goals for which to strive. On most mammograms, the pectoralis major muscle should be visible down to the level of the axis of the nipple on the MLO projection, and the distance from immediately beneath the nipple to the edge of the film on the CC view should usually be within 1 cm of the distance from under the nipple to the pectoralis major on the MLO (Fig. 13-3) (8). The breasts should be pulled upward and out, not drooping, and the trabecular structures should be separated by firm compression. Ideally, the junction of the breast with the upper abdomen should be visible. The inframammary fold should be open with no overlap of breast and upper abdomen (Fig. 13-4A). On approximately 30% of the CC projections, the pectoralis major muscle should be visible at the back of the breast (Fig. 13-4B). As digital tomosynthesis in the MLO projection replaces conventional two-dimensional mammography, it will become even more important for the technologist to be certain that she does not allow breast tissues to slip from the field of view.
X-Ray Penetration of the Tissues
To see lesions in dense fibroglandular tissue, the x-ray beam should be sufficiently energetic to penetrate these tissues. The American College of Radiology (ACR) recommends that the least penetrated tissues on a film/screen mammogram (the whitest areas) measure 1.0 or higher on a densitometer. This is a level at which structures can be seen through the dense (white) portions of the mammograms (Fig. 13-4C). There must also be sufficient penetration on a digital mammogram so that structures are also visible in the least penetrated areas.
Blur
There are many causes of blur or unsharpness on an image. Blur due to motion of the breast during the exposure is
often difficult to appreciate. Most breasts have at least one calcification, and observation of the sharpness or lack of sharpness of calcifications may indicate a motion problem. The fine linear structures of the breast are usually sharply defined. If they are not, then motion should be suspected. On rare occasions, perhaps due to edema, the fine lines of the breast are not sharply seen even though the breast has not moved, but this is the exception. As tomosynthesis techniques replace conventional 2D imaging, preventing motion becomes even more important since registration of multiple images will be compromised by movement.
often difficult to appreciate. Most breasts have at least one calcification, and observation of the sharpness or lack of sharpness of calcifications may indicate a motion problem. The fine linear structures of the breast are usually sharply defined. If they are not, then motion should be suspected. On rare occasions, perhaps due to edema, the fine lines of the breast are not sharply seen even though the breast has not moved, but this is the exception. As tomosynthesis techniques replace conventional 2D imaging, preventing motion becomes even more important since registration of multiple images will be compromised by movement.
Including “All” the Breast Tissue
Given the geometry of the breast and chest wall, it is likely that some breast tissues will be missed, no matter how well the patient is positioned. We have encouraged the manufacturers to use our idea and provide independent compression of the breast so that the detector (film/screen or digital) can be placed around the curve of the chest wall to image more breast tissue that extends beyond the edge of the compression plates. The technologist must work with the patient to urge her to stay as far into the machine as possible so that as much breast tissue can be imaged without causing undue discomfort. One manufacturer even provides a traction device, which was invented at the MGH, to assist the technologist (Planmed—Finland).
The goal of every screening mammogram should be to image as much tissue as possible with high spatial and contrast resolution. The criteria described above are optimal. Individual patients vary, and it is usually not possible to accomplish ideal positioning in every projection for all women. For example, visualization of the pectoralis major muscle on the CC view is expected in only 30% of mammograms. Although it is rare that all of the positioning criteria are met, the technologist should strive to accomplish optimal positioning with each exposure.
Artifacts
Although it is almost impossible to eliminate all artifacts, efforts should be made to reduce them to a minimum. Digital detectors with the acceptance of computed radiography (CR) are sealed so that dust should not be a problem. The screens in film/screen systems should be cleaned at least once a week, or more frequently, to eliminate dust that can block the light from the screen from reaching the film, resulting in bright specks that simulate microcalcifications. Scratches on the screen or on the film can cause pseudolesions or distract from seeing real lesions. Unusual artifacts usually have a simple explanation (Fig. 13-4D). Artifacts on digital mammograms may be more difficult to eliminate. Bad pixels may be removed electronically as long as they are not too close together, such that they eliminate too many of the detector elements in an area, obscuring significant findings. Other digital artifacts can occur from faulty electronics, and there are quality-control programs that are used to detect and eliminate these.
Mammographic Interpretation
The observer should avoid the urge to immediately view mammograms too closely. It is preferable to sit back and view the images from a distance, bringing foveal vision to bear on the entire image. Some observers find the use of a minifying lens beneficial to reduce the size of the images. This aids in appreciating areas of asymmetry.
The images should then be viewed from a distance of 1 to 1½ feet to begin evaluating some of the tissue details. Finally, the observer should evaluate the mammograms so as to view the subtle details. Although all structures visible on the mammogram can be seen by the normal, unaided eye, a magnifying glass is helpful for making small detail
more obvious. One of the advantages of digital images is that they can be electronically enlarged (the same as photographic enlargement), obviating the need for a magnifying lens. Until monitors are available that can display all of the digitally acquired imaging data, when reading digital images the observer should remember that, at some point, the images is sufficiently displayed as to provide viewing of all of the data that were acquired since some systems acquire data at a higher resolution than can be displayed over the entire breast on the monitor.
more obvious. One of the advantages of digital images is that they can be electronically enlarged (the same as photographic enlargement), obviating the need for a magnifying lens. Until monitors are available that can display all of the digitally acquired imaging data, when reading digital images the observer should remember that, at some point, the images is sufficiently displayed as to provide viewing of all of the data that were acquired since some systems acquire data at a higher resolution than can be displayed over the entire breast on the monitor.
The images should be systematically reviewed with particular attention to the deep tissues, especially near the inframammary fold and high in the axilla (places where the rare tumor may be overlooked). Because most cancers develop at the interface between the edge of the parenchyma and the subcutaneous and retromammary fat (9), these tissues
should be evaluated for architectural distortion, masses, or calcifications. Digital systems facilitate the evaluation of these zones by providing equalization algorithms that enhance these tissues, which are often “burned out” on film/screen mammograms (Fig. 13-5).
should be evaluated for architectural distortion, masses, or calcifications. Digital systems facilitate the evaluation of these zones by providing equalization algorithms that enhance these tissues, which are often “burned out” on film/screen mammograms (Fig. 13-5).
Calcifications
Calcifications are important to find. There is ongoing debate as to the importance of findng ductal carcinoma in situ (DCIS), which is virtually only found by finding calcifications on a mammogram. However, not only do calcifications often represent early intraductal cancer (DCIS), but, in a study that we undertook, we found that 17% of invasive cancers were found by mammography only because of the calcifications that had formed in the intraductal portion of these cancers. In these cases the calcifications represented the tip of the iceberg. In addition, the radiologist should be trying to find the small masses and areas of architectural distortion that are formed by invasive cancers. Data from the randomized controlled trials of mammography screening, as well as historical data and tumor modeling, show that we should be trying to find invasive cancers before they reach 1 cm in size. Invasive cancers are potentially lethal, and prognosis is improved if they can be treated while they are ≤1 cm in size (10,11,12). In general, the likelihood of metastatic spread is a probabilistic phenomenon that is based on the number of cells that make up the cancer. The likelihood of metastasis begins to increase rapidly for tumors when they become larger than 1 cm (13). The analysis of the mammogram should include a search for
small masses and areas of architectural distortion, as well as calcifications.
small masses and areas of architectural distortion, as well as calcifications.
Review of Old Films
If old films are available, comparison is important to search for any changes. If the patient has been studied previously at another center, she should be urged to obtain and bring her old films with her at the time of her examination. If she does not bring these films, an effort should be made to obtain them if the present study suggests an abnormality. If the present study is unremarkable, obtaining outside films adds little advantage. Bassett et al (14), in a review of 1,432 cases in which 87% had previous films, found that clinical management was altered in 35 of the 1,093 cases for which the previous films were located. Of the seven biopsies that were performed as a result, two cases of cancer would have been missed without the previous mammograms. The two cancers were detected because of changes between mammograms from their own facility. Their estimated cost for obtaining the 134 outside studies, of the 274 that they requested, amounted to $12.52 per study (postage and labor), with an average delay of 43 days before the outside studies arrived. The outside studies resulted in the avoidance of only six workups and one biopsy. Although, in the ideal world it might be useful to have all previous studies, given the difficult logistics of obtaining outside studies, the delays involved, the cost, and the marginal benefit (as well as the frequency with which loaned films are lost), we confine our efforts to obtaining outside films when there is a management need for them. As noted, we request that all women obtain their outside studies and bring them in to their appointment, but, if the mammogram is negative, we do not routinely seek outside films. The bottom line is that obtaining outside studies, when the present study is unremarkable, is not a standard of care.
Neodensities
Fortunately, the mammographic appearance of the breast is fairly stable from year to year, and the overall percentage of attenuating tissues decreases in many women (not all) with increasing age. Changes, such as the development of new masses (neodensities) or new calcifications, are fairly uncommon so that when they do appear they should be analyzed to determine their significance. Sumkin et al (6) suggested that mammograms need only be compared to the study from the previous year. Unfortunately, their study design missed important factors (15). It is a good idea to compare studies that are at least 2 years apart because changes may not be as apparent from one year to the next (Fig. 13-6). Comparing images to 1 year prior increases the chance that a slowly developing change will be overlooked from year to year. A 2-year (or longer) time difference makes these subtle changes more apparent.
In general, a new focal abnormality warrants careful review and often requires ultrasound (for cyst/solid differentiation) or tissue evaluation (needle biopsy or open biopsy), as needed to make a diagnosis. The development of multiple (three or more) similar findings in noncontiguous areas usually represents a benign change. These are almost always cysts, and the development of multiple (three or more) rounded densities usually does not require additional evaluation.
Analyzing Lesions Detected by Mammography
Problems can be avoided by following a simple approach to the image interpretation:
Find it.
Is it real?
Where is it?
What is it?
What should be done about it?
These five simple rules, followed in order, provide an organized framework for image evaluation.