Intramedullary spinal cord tumors are rare neoplasms that can result in significant patient morbidity and mortality. Symptom onset is often insidious and not specific; thus, imaging is imperative for accurate diagnosis and localization of these tumors. Radiologists should be familiar with the key MR imaging features of common and unusual intramedullary tumors, in order to identify the most likely diagnosis or at a minimum develop an accurate differential diagnosis as this in turn will guide management.
Key points
- •
Intramedullary spinal cord tumors are uncommon tumors of the central nervous system, with ependymomas and astrocytomas comprising the vast majority of these lesions.
- •
Clinical presentation is often challenging as symptoms are insidious, vague and nonspecific, and therefore imaging is essential for diagnosis and localization of cord tumors.
- •
Biopsy of spinal cord lesions is considered a high-risk procedure that carries a substantial risk of potential permanent neurologic sequelae.
- •
Specific MR imaging characteristics can help to distinguish different types of intramedullary tumors from one another, and from secondary lesions of the spinal cord.
Introduction
Primary tumors of the spinal cord are rare, comprising only 4% to 8% of tumors of the central nervous system (CNS). These tumors are classically grouped by anatomic location: extradural, intradural-extramedullary, and intramedullary with decreasing order of prevalence, respectively. Extradural tumors are most commonly metastases arising from the osseous vertebral column. Intradural-extramedullary tumors are usually either meningiomas or nerve sheath tumors (eg, neurofibromas or schwannomas). This article will focus on the least common type of spinal neoplasms, intramedullary tumors, which account for 8% to 10% of primary spinal cord tumors, and arise from the parenchyma of the spinal cord itself. , The most common intramedullary tumors are glial in origin and include ependymomas and astrocytomas, accounting for 70% to 80% of all intramedullary neoplasms, followed by hemangioblastomas. Other less frequent cord tumors will be briefly discussed as well.
Clinically, intramedullary lesions pose a tremendous challenge for patients and clinicians as their eloquent location can lead to significant neurologic impairment. At initial presentation, symptoms are frequently insidious and nonspecific, presenting as subacute back pain, myelopathy, or radiculopathy. MR imaging is the imaging modality of choice for detection, localization, and careful preoperative planning of these tumors. As spinal cord biopsies carry a substantial risk of worsening neurologic dysfunction, imaging characterization is critical in guiding surgical management and patient counseling. While it is understood that definitive diagnosis of intramedullary neoplasms on imaging may not be always possible, providing an informed, narrow differential diagnosis based on key differentiating imaging features remains an integral part of standard clinical practice.
Normal anatomy and imaging technique
An understanding of the anatomic compartments of the spine described above is paramount when evaluating and localizing an abnormality to the intramedullary space. Some hallmark features of intramedullary neoplasms (in particular glial neoplasms) include spinal cord expansion, the presence of contrast-enhancement, and cysts (whether tumoral or nontumoral). Importantly, some or all of these features may be found in nonneoplastic lesions of the spinal cord, and therefore the presence of these imaging characteristics should not preclude consideration of other potential diagnoses. If expansion of the spinal cord is not present, however, other etiologies such as inflammatory and nonneoplastic conditions should be more strongly considered.
Imaging Protocols
MR imaging of the spine with and without contrast is the imaging test of choice for evaluating intramedullary spinal tumors. Spinal computed tomography (CT) poorly evaluates the spinal cord and provides insufficient information to suggest a useful differential diagnosis or determine extent of involvement. If MR imaging is contraindicated, CT myelography can be used to assess for spinal cord expansion; however, this technique may be insensitive for early or small lesions that do not grossly expand the spinal cord and does not provide characterization of spinal cord lesions even when they do result in spinal cord expansion. Thus, CT is of very limited value in the workup of spinal cord tumors.
Standard MR imaging protocol for evaluation of spinal cord tumors includes precontrast and postcontrast T1-weighted and T2-weighted sequences in both sagittal and axial planes. Postcontrast sequences are imperative for the diagnosis of these lesions. Advanced MR imaging techniques have recently emerged as potentially helpful tools for distinguishing between types of intramedullary neoplasms and also for defining the extent of the lesions. These techniques, including MR spectroscopy, diffusion weighted imaging, diffusion tensor imaging (DTI), and phase contrast imaging are not standard tools used in the clinical workspace as their practical utility has yet to be clearly defined. DTI has shown promise in distinguishing between spinal cord neoplasms and inflammatory lesions, with tumors demonstrating significantly lower fractional anisotropy levels compared with inflammatory lesions, reflecting interruption of the normal highly anisotropic, longitudinally oriented white matter tracts in neoplasms. The following discussion and imaging review will center around imaging features on standard MR imaging acquisitions.
Imaging findings
Primary Glial Neoplasms
Ependymomas
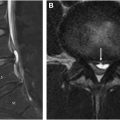
Ependymomas are the most common primary spinal cord neoplasm in adults, accounting for 50% to 60% of all intramedullary tumors. They are considered slow growing tumors arising from the ependymal cells of the central spinal canal and often present with insidious symptoms of back pain and dysesthesias that can precede diagnosis by 3 to 4 years. Spinal ependymomas are more common in patients with neurofibromatosis 2 (NF2), affecting approximately 33% to 53% of individuals with NF2, and in this instance, they are frequently multiple and demonstrate a string of pearls appearance throughout the spinal cord and cauda equina ( Fig. 1 ).

Significant changes to the histologic classification of ependymomas were made in the most recent World Health Organization (WHO) CNS tumor update in 2021 that reclassified ependymomas according to their neuroanatomic location with spinal ependymomas now recognized as a discrete tumor type. Myxopapillary ependymomas and subependymomas are exceptions as they can arise anywhere along the neuraxis, including the spinal cord as discussed below. Additionally, as molecular tumor biology has begun to play a central role in diagnosis and management of CNS tumors, MYCN-amplified spinal ependymomas are newly recognized as a discrete, more aggressive tumor type with anaplastic histology and poor prognosis.
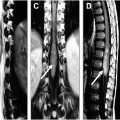
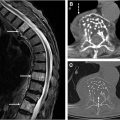
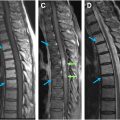
Imaging findings of ependymomas correlate well with their anatomic origin along the central canal of the spinal cord, classically appearing as a well-defined, expansile mass located centrally within the spinal cord. Signal characteristics include T1 hypointensity to isointensity, T2 isointensity to hyperintensity, and variable enhancement patterns, although some degree of enhancement is nearly always present. Cystic and hemorrhagic components are common with classically described polar cysts (nontumoral cysts at the tumor margin) seen in the majority of spinal ependymomas, and the hemosiderin cap sign (T2 hypointense signal at the superior or inferior tumor margin) seen in 20% to 33% percent of cases and highly predictive of ependymoma ( Fig. 2 ). Surrounding edema and syrinx (syringohydromyelia) are often seen, likely related to disruption of normal cerebrospinal fluid (CSF) flow. These tumors most commonly present in the cervical spine ( Fig. 3 ), although they can arise in the thoracic segment, and rarely, in the lumbar spine.


Maximal safe surgical resection is the first-line treatment for spinal ependymomas with gross total resection (GTR) offering the best prognosis. Lower grade spinal ependymomas (WHO grade 1 and 2) often have a distinct tumor capsule that facilitates the preservation of a clear dissection plane and correlates with increased potential for GTR and fewer postoperative neurologic deficits. En bloc resection is ideal, when possible, to avoid CSF dissemination. Postoperative radiotherapy should be offered to all patients with WHO grade 3 and patients with WHO grade 2 ependymomas with subtotal resection. Patients with tumor recurrence that is no longer eligible for local treatment may benefit from chemotherapy, specifically temozolomide in adults.
Overall, spinal ependymomas have a favorable prognosis with 10-year overall survival rates of 72% in children and greater than 90% in adults. MYCN-amplified spinal ependymomas demonstrate a uniquely poor prognosis with frequent early relapse (recurrence rate of 75%–100%), metastasis with leptomeningeal spread, and limited therapeutic response with overall survival of 87 months (in a small 13 case series).
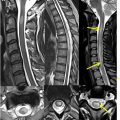
Myxopapillary ependymomas are thought to arise from the ependymal cells at the filum terminale and, as such, nearly all cases present either as an intradural, extramedullary mass in the lumbar spine, or as an intramedullary mass in the conus medullaris. These tumors were recently upgraded from grade 1 to grade 2 due to their likelihood of recurrence, which is now understood to be similar to other spinal ependymomas. On imaging, they classically appear as well-defined oval or sausage-shaped lesions that fill the distal spinal canal and can scallop the vertebral bodies, due to their gradual exophytic growth. Areas of internal hemorrhage, cystic degeneration, and calcification can result in heterogenous signal characteristics ( Fig. 4 ).

Subependymomas (WHO grade 1) are extremely rare in the spine but have a unique imaging appearance that is distinct from other ependymal tumors and astrocytomas. They present with extremely steep or tapered swelling of the spinal cord at the margins of the lesion on sagittal MR imaging sequences, referred to as the bamboo leaf sign . This is seen in 76% of subependymomas and is thought to correspond to its eccentric subpial location noted at surgery.
Astrocytomas
Astrocytomas of the spinal cord, while rare, are the most common intramedullary tumor of the pediatric and young adult populations, accounting for 90% of primary spinal cord tumors in children under the age of 10 and 60% in adolescents. In adults, spinal astrocytomas comprise 30% of intramedullary spinal tumors. While the majority of astrocytomas in young patients are low grade, those that occur in older patients are more often high-grade and portend a poor prognosis. , These tumors do have a genetic association with neurofibromatosis 1 (NF 1); in patients with NF 1 who develop spinal astrocytomas, a statistically higher number are male. As with other intramedullary lesions, these tumors often present with vague clinical symptoms; however, it should be noted that otherwise healthy children presenting with insidious back pain or painful scoliosis warrant a full and thorough evaluation with imaging.
On imaging, astrocytomas of the spinal cord are most commonly located in the cervical greater than thoracic region and demonstrate fusiform expansion of the cord, often extending multiple vertebral segments. They often either have an eccentric appearance within the cord or can demonstrate holocord involvement (in the case of pilocytic astrocytomas). This appearance is the result of tumor diffusely infiltrating the spinal cord parenchyma rather than the central ependymal lining, which can be contrasted with the circumscribed nature of spinal ependymomas. Enhancement characteristics vary both in degree and longitudinal extent, ranging from absent to large areas of heterogeneous enhancement, and tend to accentuate the infiltrative nature of the lesions. The presence or absence of enhancement, however, does not help distinguish between high-grade and low-grade tumors. Cystic areas may be intratumoral with peripheral enhancement or could be found along the cranial and caudal margins of the tumor without demonstrable enhancement; the latter typically called peritumoral or polar cysts. Hemorrhage is typically absent; a small minority of cases may have hemorrhagic components ( Figs. 5 and 6 ).



Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree