Iodinated Radiocontrast Agents
Ibrahim Shah
Kevin L. Berger
George S. Abela
Radiocontrast agents are compounds used to improve the visibility of internal bodily structures in an X-ray image. Iodine, because of its high atomic number, effectively absorbs X-rays and appears radio-opaque in an X-ray image. Since iodine-based contrasts are used in computed tomography angiography (CTA), the focus of this chapter is iodine-based contrast media.
Classification
In 1929 the first organic iodide preparation (Selectan) was explored, which contained one iodine atom per benzoic acid ring. All iodinated radiocontrast agents are derivatives of benzoic acid, but the number of iodine molecules, the ionic and osmolar composition, and the viscosity vary among different agents. Iodinated contrast agents are divided into those that ionize in solution (ionic agents) and those that do not (nonionic agents) (Table 5.8).
TABLE 5.8 Classification of Contrast Media | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
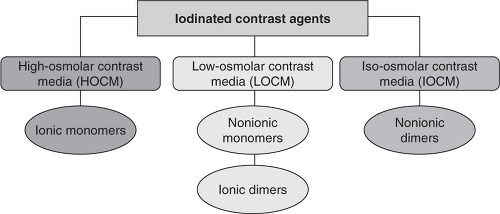
For CTA, the usual amount of contrast agent used is about 100 mL and an iodine concentration of 320–370 mg/mL is required for left ventricular and coronary contrast injection. The higher the number of iodine molecules attached to a benzene ring, the lower the concentration of the solution needed to be an effective radiocontrast, and hence the lower the osmolality. These agents are divided into three categories on the basis of osmolality (Figure 5.11):
High-osmolar contrast media (HOCM)
Low-osmolar contrast media (LOCM)
Iso-osmolar contrast media (IOCM)
High-Osmolar Contrast Media
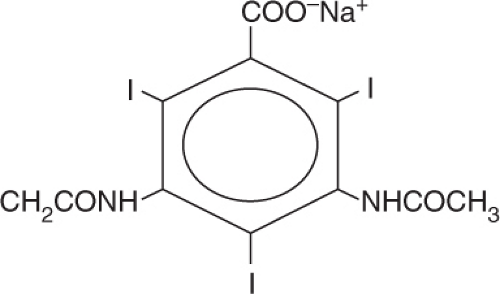
These agents are monomeric salts of tri-iodinated benzoic acid. The iodine atoms are attached at position 2, 4, 6 of the benzene ring and position 1 has a cation, either sodium or methylglucamine (Figure 5.12).
These agents contain three iodine atoms for every two osmotically active ions, i.e., the substituted benzoic acid ring and the cation at position 1, and thus are considered ratio 1.5 agents. They are markedly hypertonic with an osmolality >1500 mOsm or approximately six times higher than plasma.
Renografin contains sodium citrate and sodium ethylenediaminetetraacetic acid (EDTA) as additives, which has calcium-binding properties. Angiovist, on the other hand, has calcium EDTA as its additive to overcome the calcium-binding effects.
The hypertonicity and calcium-chelating properties of these compounds contribute to their side effects.1
Low-Osmolar Contrast Media
These agents have three atoms of iodine for each ion. Being a ratio of 3:1, they are only two to three times the osmolality of plasma. Hence, they are referred to as low-osmolar contrast agents (LOCM). These agents are either nonionic monomers or ionic dimers. There is some evidence that LOCM produce fewer allergic side effects and are less nephrotoxic, but this has been difficult to confirm in large clinical studies.2
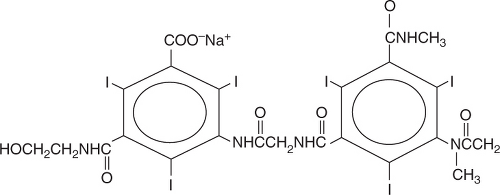
Ionic Dimers
An ionic dimer is created by the formation of a dimer in which six iodine atoms are associated with two osmotically active agents and hence are ratio 3 (Figure 5.13). The osmolality is
substantially reduced (~600 mOsm/kg). Because of the low osmolality of these agents, they have fewer side effects.3 An example is ioxaglate (Hexabrix).
substantially reduced (~600 mOsm/kg). Because of the low osmolality of these agents, they have fewer side effects.3 An example is ioxaglate (Hexabrix).
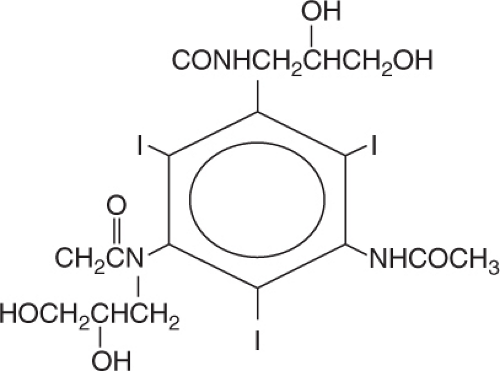
Nonionic Monomers
This group includes iohexol (Omnipaque), iopamidol (Isovue), and ioversol (Optiray). These agents are the most commonly used. Nonionic agents have three iodine atoms for each osmotically active particle (Figure 5.14). These agents are water soluble in a noncharged form (i.e., without an associated cation), and hence provide an equivalent amount of iodine as the ionic counterparts with lower osmolality.4
Iso-osmolar Contrast Media
Nonionic Dimers
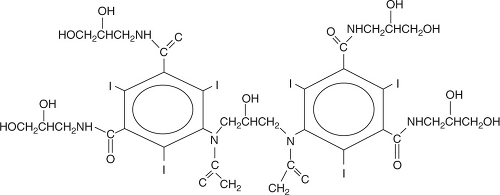
This class of contrast agents has osmolality similar to that of plasma. They are nonionic dimers and contain six atoms of iodine per dimer and hence are ratio 6 agents (Figure 5.15). Iodixanol (Visipaque) is the only agent currently in this class. IOCM has the potential of causing fewer side effects in patients undergoing interventional procedures,2 and its use is associated with fewer adverse cardiac events compared with LOCM.2,3
Although IOCM have been postulated to produce less cardioreflex activity, a recent randomized control trial found no differences in heart rate parameters between non-ionic LOCM and IOCM when using the same injection rate and total iodine dose.4 There is also data suggesting a reduction in nephrotoxicity with this agent, although the magnitude of this benefit is still unresolved.5 However, in the IMPACT study, a prospective trial failed to identify any difference in the incidence of contrast induced nephropathy between equal iodine doses of non-ionic LOCM and IOCM in patients with pre-existing stable chronically reduced kidney function.6
Adverse Effects of Contrast Agents
In general, nonionic compounds have fewer side effects as they do not dissociate into component molecules. The side effect profile is also dependent upon the osmolality of the compound, such that the hyperosmolar solutions have more side effects (Table 5.9).
TABLE 5.9 Adverse Effects of Contrast Agents Can Be Grouped into the Following Categories | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Anaphylactoid reactions are immediate type reactions and are triggered by activation of basophils and macrophages in the lungs. This can cause rapid vasodilatation and circulatory collapse. It has also been reported that the intravenous use of iodinated contrast agents is associated with more events than the intra-arterial use, since the contrast given intravenously courses through lungs where it can trigger the cell activation. In distinction, late contrast reactions occur hours to days later.7,8 The delayed skin reaction is a T-lymphocyte-mediated delayed hypersensitivity.9
Symptoms of delayed reactions may also resemble a flu-like syndrome and include fever, chills, nausea, vomiting, abdominal pain, fatigue, and congestion.10
Toxic reactions are caused by the inherent physical or chemical properties of contrast agents or the additives necessary for their stabilization. Most toxic effects are thought to result from the hyperosmolality of these agents.
Reaction to radiocontrast agents are classified according to their severity:
o Mild (grade I: single episode of emesis, nausea, sneezing, or vertigo)
o Moderate (grade II: hives, multiple episodes of emesis, fevers, or chills)
Severe (grade III: clinical shock, bronchospasm, laryngospasm or edema, loss of consciousness, hypotension, hypertension, cardiac arrhythmias, angioedema, or pulmonary edema)
The reported incidence of severe reactions is about 1:1000,11




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree