Abstract
Kearns-Sayre Syndrome (KSS) is a rare multisystem mitochondrial disorder affecting muscles, the central nervous system, and the endocrine system. KSS is characterized by T2WI/FLAIR hyperintensities in the subcortical white matter, brainstem, globi pallidi, thalami, and middle cerebellar peduncles. Here, we report a case of KSS with extraocular muscle atrophy in which MRI performed approximately 10 years after the initial MRI examination revealed lesion expansion and diffusion restriction of subcortical white matter affecting U-fibers.
Introduction
Chronic progressive external ophthalmoplegia (CPEO) and Kearns-Sayre Syndrome (KSS) are caused by mutations in mitochondrial DNA and appear to represent a clinical continuum: CPEO is a disease characterized by ptosis and external ophthalmoplegia, whereas KSS is a multisystem disorder characterized by onset before age 20 years, pigmentary retinopathy, and external ophthalmoplegia [ ]. In addition, the diagnosis of KSS requires the presence of at least one of the following: cardiac conduction abnormalities, cerebellar ataxia, cerebrospinal fluid (CSF) protein levels ≥100 mg/dl, short stature, endocrine abnormalities, or cognitive decline [ ]. MRI findings in young adults and children with KSS have been reported to be characterized by T2-weighted imaging (T2WI)/FLAIR hyperintensities in the subcortical white matter and one or more regions of the brainstem, globi pallidi, thalami, and middle cerebellar peduncles [ , ].
KSS is a rare disease, and there are no reports of long-term imaging follow-up studies beyond 10 years. Here, we report a case of KSS in which significant changes in MRI findings were observed 10 years after the initial MRI.
Case presentation
An 8-year-old boy visited a hospital with a chief complaint of right eyelid ptosis for the past year. Regarding family history, his grandfather had myasthenia gravis, but there was no family history of mitochondrial disease. At that time, only right inferior oblique muscle overaction was present and no other abnormalities were noted. Two years later, he presented with the complaint of bilateral ptosis and muscle weakness, and was referred to our hospital for brain MRI examination. MRI was performed using a 1.5T MR scanner (Achieva, Philips Healthcare, Best, The Netherlands). FLAIR showed mild cerebral atrophy and high signal areas in the left caudate nucleus and dorsal midbrain ( Fig. 1 ), and severe atrophy of extraocular muscles was found on the both sides ( Fig. 2 ). No diffusion restriction was observed, and no abnormal signals were found in the subcortical white matter, middle cerebellar peduncle, or cerebellum. He had ptosis, restricted eye movement, short stature and hearing loss, and CSF showed high lactate and pyruvate levels. As a result, a tentative diagnosis of mitochondrial disease was made. At the age of 11, a muscle biopsy on his left arm revealed red ragged fibers, and mitochondrial DNA sequence analysis revealed a large deletion of approximately 4 kB, leading to a diagnosis of CPEO. Treatment with fursultiamine and ubidecarenone was initiated. At the age of 14, retinitis pigmentosa was pointed out, confirming the diagnosis of KSS. At the age of 15, he enrolled in a clinical trial of 5-ALA treatment for Leigh encephalopathy. Although the treatment had little effect, he continued it at his own request. He developed diabetes mellitus at the age of 18.


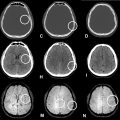
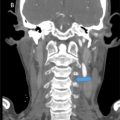
The patient was referred to our hospital at the age of 20 with almost no eye movement. He had slight paralysis on the left side of his face and difficulty protruding his tongue. Muscle atrophy was evident in his lower limbs, making it difficult for him to stand or walk. His muscle tone was generally increased, and his limbs were rigid. He had no sensory or autonomic symptoms. Laboratory analysis revealed diabetes (blood glucose 121mg/dL; normal: 73-109 mg/dL, HbA1c 7.1%; normal: 4.9%-6.0%, C-peptide 3.5ng/mL; normal:0.8-2.5ng/mL), hypercholesterolemia (total cholesterol 268mg/dL normal:142-248mg/dL), and liver dysfunction (AST:53U/L; normal:13-30U/L, ALT:86U/L; normal:10-42U/L, γ-GTP:76U/L; normal:13-64U/L, ALP 148U/L; normal:38-113U/L, cholinesterase 268U/L; normal:142-248, LDH:256U/L; normal:124-222U/L). Echocardiography revealed no morphological or functional abnormalities, and left ventricular contractility was at the lower limit of the normal range. An electrocardiogram showed complete right bundle branch block and left axis deviation. MRI was performed using a 3T MR scanner (Ingenia, Philips Healthcare, Best, The Netherlands). Brain MRI showed severe atrophy of the cerebrum, brainstem, and cerebellum, which had worsened over the past 10 years ( Fig. 3 ). All extraocular muscles were thinning ( Fig. 4 ). 3D FLAIR showed hyperintensities in the left caudate nucleus, bilateral globi pallidi, pyramidal tracts, middle cerebellar peduncles, paravermal areas, brainstem, and bilateral subcortical white matter including U-fibers with sparing periventricular regions ( Fig. 3 ). DWI showed restricted diffusion in these lesions ( Fig. 3 ). Contrast-enhanced MRI was never performed in this patient.