The coronavirus disease 2019 (COVID-19) pandemic has led to a variety of health challenges, with “long COVID” emerging as a widespread and debilitating post-acute syndrome among a considerable number of infected patients. This PET review synthesizes current evidence of the neurologic and psychiatric sequelae of COVID. This review also explores the pathophysiological mechanisms of these results, including astrocyte dysfunction and glutamate dysregulation, as well as the multimodal comparison to MR imaging findings. The findings underscore the potential for long-term brain injury. Additionally, the authors discuss the role of advanced imaging multimodal techniques in diagnosing, monitoring, and guiding treatment strategies for long COVID.
Key points
- •
Neurological and Psychiatric Impact of Long COVID: Long COVID is associated with persistent neurological symptoms, such as brain fog, fatigue, and cognitive impairment, as well as psychiatric symptoms like anxiety and depression, significantly affecting quality of life.
- •
Brain Hypometabolism: PET imaging reveals reduced glucose metabolism in key brain regions, indicating long-term impacts on cognitive and emotional regulation.
- •
Hypothesis: Neuroinflammation and Brain Dysfunction: Viral persistence, immune dysregulation, and blood-brain barrier disruption may trigger neuroinflammation, marked by microglial activation and glutamatergic dysfunction, leading to long COVID’s cognitive and neurological symptoms.
- •
Importance of Continued Research: Further studies, especially large-scale longitudinal research, are essential to fully understand long COVID’s long-term effects on brain health, especially the neurodegeneration risk, and to develop targeted interventions.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has had a profound and widespread impact on global health. Initially marked by severe acute respiratory syndrome, the pandemic has unveiled a broader range of prolonged symptoms known collectively as “long COVID.” This complex multisystem disorder can affect nearly every organ, with possible severe disability. The condition represents the constellation of post-acute and long-term health effects caused by SARS-CoV-2 infection. Long COVID affects approximately 15% of infected individuals, leading to a variety of persistent symptoms including fatigue, cognitive impairment, and cardiorespiratory troubles that can last for months after the acute phase of the illness.
Full recovery from long COVID appears to be uncommon, with only 7% to 10% of the diseased population reporting such improvement after 2 years. In fact, some symptoms may become lifelong issues, drastically affecting patients’ well-being, work, and social life. By the end of 2023, the global incidence of long COVID was estimated to have reached around 400 million cases with cases being most prevalent among women aged 30 to 50 years and the elderly. Initial disease severity and the number of reported acute symptoms are predictive of the extent of prolonged health issues, though most cases of long COVID occur in those with mild initial illness. At the health system level, long COVID strains resources and increases costs due to the need for ongoing specialized care. Long COVID contributes to reduced labor participation, productivity losses, and increased disability, translating to an estimated annual global impact of around $1 trillion, corresponding to about 1% of the global economy.
Among the various long-lasting symptoms, the potential impact on brain health, particularly the cognitive impairment, is of major concern. , Often referred to as “brain fog”, this cognitive impairment manifests as difficulties in concentration, memory loss, and reduced mental clarity, which severely impact daily functioning and quality of life. Of note, and beyond the cognitive impairment, many symptoms of long COVID could be explained by a brain substrate: the loss of smell and taste, sleep disturbances, pain, emotion disorders, and other symptoms that might be related to dysautonomia, such as breathlessness, tachycardia, and orthostatic intolerance. This possible brain impairment in a significant subset of COVID-19-infected patients also raises concerns about the potential impact on neurodegenerative diseases that could emerge. Given the established association between brain injuries, and specifically those linked with viral infections, and an increased risk of conditions like Alzheimer’s disease, the brain changes observed in patients with COVID-19 may similarly elevate the risk of dementia and other neurodegenerative disorders.
All such has motivated the World Health Organization to recognize long COVID as a public health concern, emphasizing the need for research into its pathophysiology. Several key mechanisms have been proposed, including viral persistence, immune dysregulation, mitochondrial dysfunction, complement dysregulation, endothelial inflammation, blood–brain barrier dysfunction, and microbiome dysbiosis. These mechanisms likely interact and shape the diverse clinical manifestations. They enhance the broader recognition that post-acute and chronic illnesses often emerge as a long tail of infectious disease outbreaks, previously described as post-acute infection syndromes. ,
Molecular imaging with PET could help elucidate these biologic mechanisms and contribute to improved diagnostics aiding in diagnosis, risk stratification, and treatment monitoring of long COVID. Moreover, visualizing imaging evidence of long COVID could help promote the recognition of the disorder and combat possible. This article aims to synthesize current knowledge on the impacts of COVID-19 on the brain among patients with long-lasting neurologic and psychiatric symptoms. , Drawing on PET studies of long COVID, and complementary on MR imaging studies, the authors summarize the proposed underlying pathophysiological mechanisms, and to specify the potential clinical value of molecular neuroimaging. ,
Brain impairment in long coronavirus disease: building the hypothesis
The potential long-lasting effects of COVID-19 on the brain can be inferred from COVID-19 studies showing possible neurologic and psychiatric complications in the acute stages of the disease, and from postinfectious studies showing long-lasting effects on the brain from other viruses.
Acute Brain Complications of Coronavirus Disease 2019
Several neurologic complications have been highlighted in the initial phase of COVID-19, suggesting direct or indirect neurotropism of the virus. SARS-CoV-2 has been, for example, associated with an increased risk of stroke, particularly in severe cases. The virus can lead to clotting abnormalities, which lead to ischemic strokes. These strokes can occur in young patients without typical risk factors, indicating a direct or indirect impact of the virus on the cerebrovascular system. Another significant acute complication of COVID-19 is encephalitis. This condition can again result from the direct invasion of the central nervous system by the virus, or from an abnormal immune response to the infection. Additionally, COVID-19 can cause a range of other acute neurologic symptoms, including Guillain–Barré syndrome (GBS). GBS is a rare immune-mediated acute polyradiculoneuritis in which the immune system attacks the nerves, leading to muscle weakness and, in severe cases, paralysis. Overall, these acute neurologic effects illustrate the possible direct or indirect effects of COVID-19 on the nervous system.
Long-Term Neurologic and Psychiatric Impacts from Other Viral Infections
Long-lasting impacts on the brain of SARS-CoV-2 are suggested by previous studies on postinfectious effects caused by other viruses. Experiences with Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS), in particular, indicate that coronaviruses can have enduring effects on brain health, with both neurologic and psychiatric symptoms. While the full extent of these impacts is still under investigation, it is likely that coronaviruses, like other neurotropic viruses, have the potential to cause persistent and significant pathophysiological changes to the brain in survivors. In developing brains, the long-term neurologic impact of viral infections can be particularly severe. For instance, the Zika virus, which caused widespread developmental delays among newborns during the 2015 outbreak, demonstrated how viral infections can lead to serious neurologic issues, including microcephaly and cognitive delays. These effects are thought to result from both direct viral invasion of the central nervous system and the inflammatory responses triggered by the infection. In the longer term, viral infections could also act as triggers for neurodegenerative diseases, such as Alzheimer’s and Parkinson’s diseases, possibly accelerating the onset of these diseases in predisposed individuals. , , , For example, the presence of certain viruses has been linked to increased amyloid-beta deposition in Alzheimer’s disease.
In addition to neurologic outcomes, viruses can also have significant psychiatric consequences. Adults who have survived previous coronavirus infections could have reported chronic psychiatric symptoms, including anxiety, depression, and posttraumatic stress disorder (PTSD). These symptoms may be exacerbated by the stress and trauma associated with the infection and the broader societal impacts of the pandemic, such as isolation and financial strain. Research suggests that viral infections may disrupt the normal functioning of the brain’s neurotransmitter systems, leading to these psychiatric symptoms. Children can also suffer from long-term psychiatric effects following viral infections. , The experience of illness, hospitalization, and separation from family can be traumatic, leading to increased rates of anxiety, depression, and behavioral problems. Additionally, the potential for neuroinflammation and direct viral effects on the brain may predispose to psychiatric disorders later in life.
The Historical Example of the Spanish Flu
The most significant historic example of long-term neurologic and psychiatric effects of viral infections is certainly provided by the 1918 Spanish flu pandemic. , Caused by the H1N1 influenza virus, it had a profound and lasting impact on global health, with its repercussions extending far beyond the acute phase of the illness. This pandemic not only claimed millions of lives but also left many survivors with enduring neurologic and psychiatric sequelae, providing a case study in understanding the potential long-term effects of viral infections on the brain.
One of the most striking neurologic complications associated with the Spanish flu was the development of encephalitis lethargica, also known as “sleeping sickness.” This condition was characterized by a wide range of symptoms, including lethargy, movement disorders, and, in severe cases, coma. Encephalitis lethargica led to a significant number of deaths and left many survivors with permanent neurologic damage, including Parkinsonism. The connection between the Spanish flu and encephalitis lethargica remains a subject of debate, but the temporal association and the overlapping symptoms have led many researchers to suspect that the influenza virus may have played a role in triggering this condition, either through direct viral invasion of the central nervous system or by provoking an aberrant autoimmune response that affected the brain. In addition to encephalitis lethargica, many survivors of the Spanish flu experienced a range of other neurologic symptoms that persisted long after the acute infection had resolved. These included chronic headaches, dizziness, and a general sense of malaise, which were often described as part of a “post-influenza syndrome.”
The psychiatric sequelae of the Spanish flu were also notable. Survivors of the pandemic reported high rates of psychiatric disorders, including anxiety, depression, and psychosis. There were widespread accounts of individuals who, having recovered from the flu, exhibited significant changes in personality, mood, and behavior. Some patients developed what was then referred to as “neurasthenia,” a condition marked by fatigue, irritability, and emotional instability, which, today, might be classified as a mood disorder. The relationship between the Spanish flu and psychiatric disorders is complex and likely multifactorial. On one hand, the direct effects of the virus on the brain, through either infection or inflammation, may have contributed to the development of psychiatric symptoms. Alternatively, the immense psychological stress of living through a pandemic—widespread death, uncertainty of survival, social ramifications, and economic disruptions—undoubtedly played a significant role in the observed mental health outcomes. The pandemic also occurred during a time when mental health support was rudimentary, exacerbating the long-term psychiatric toll.
The Clinical Model of Chronic Fatigue Syndrome and Myalgic Encephalomyelitis
More recently, postinfectious syndromes have been considered in the framework of chronic fatigue syndrome (CFS) and myalgic encephalomyelitis (ME) , that could also be extended to long COVID. These conditions share common features, such as debilitating fatigue, cognitive impairment, and a range of other symptoms, that can severely impact quality of life such as post-exertional malaise.
The pathophysiological mechanisms underlying these conditions are still being explored, but they may involve immune dysregulation, neuroinflammation, and metabolic dysfunction. The immune response triggered by the inciting virus may be characterized by an excessive immune response, leading to chronic inflammation and altered neurochemical signaling. These processes may then contribute to persistent fatigue and cognitive impairments as is seen in other viral infections that precede CFS/ME, such as Epstein–Barr virus or influenza.
Neuroimaging studies have provided valuable insights into the neurologic and metabolic changes associated with CFS and ME (for review, see Ref ). Functional MR (fMR) imaging scans have shown altered brain activity patterns in patients with CFS/ME, such as abnormal activation in regions related to cognitive processing like attention and memory. Both conditions may involve metabolic dysregulation affecting brain energy production and utilization. This hypometabolic state may reflect underlying neuroinflammation or metabolic dysfunction. Fluorodeoxyglucose (FDG)-PET imaging has revealed hypometabolism in specific brain regions, such as the frontal and temporal lobes, which are linked to cognitive function and emotional regulation. PET studies using translocator protein (TSPO) targets tracers like [11C] PK11195 have detected elevated microglial activation, suggesting a role for neuroinflammation in CFS/ME. This supports the autoimmune hypothesis that chronic inflammation, activation of microglia, and release of pro-inflammatory cytokines contribute to the persistent fatigue and cognitive impairments experienced by patients.
This potential link among long COVID, CFS, and ME underscores the complexity of post-viral syndromes and their impact on patient health. The overlapping symptoms could share pathophysiological mechanisms and neuroimaging signatures. Continued research is essential to further elucidate these connections.
PET neuroimaging in long coronavirus disease
Fluorodeoxyglucose Brain PET Findings
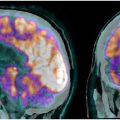
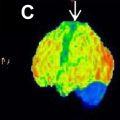
One of the key tools in understanding the neurologic and psychiatric impacts of COVID-19 has been neuroimaging, particularly the use of PET with the glucose analog 18F-fluorodeoxyglucose (18F-FDG). , Its ability to visualize metabolic activity provides critical insights into brain function and pathology, making it a valuable tool for clinicians in diagnosing and managing various brain disorders, including neurodegenerative diseases, epilepsy, brain tumors, and inflammatory conditions. , In patients with long COVID, 18F-FDG PET scans have consistently revealed a characteristic pattern of decreased glucose metabolism in specific brain regions. , , , This individual hypometabolic profile has been observed in both adults and children.
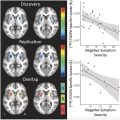
Numerous FDG-PET studies have identified significant cerebral anomalies in adults suffering from long COVID. , The first retrospective analysis of brain PET scans revealed hypometabolism in several key brain regions, including the olfactory gyrus and more broadly the limbic and paralimbic regions, the brainstem and cerebellum ( Fig. 1 , Table 1 ). These findings of brain hypometabolism were supported by multiple monocentric case-control studies and a larger multicenter study, reinforcing the across different populations and settings.

| Study Reference | Study Type | N | Age | Post-COVID Delay | Symptoms | Analysis Method | PET Hypometabolism | Link to Symptoms |
|---|---|---|---|---|---|---|---|---|
| Guedj et al, 2021 France | Monocentric retrospective | 35 | 55 y (mean) | 96 d (mean) | Fatigue (n = 35), hypo/anosmia (10), hypo/ageusia (9), cognitive complaints (17), pain (23), dyspnea (28), sleep disturbances (16) | Individual and group semiquantitative statistical parametric mapping (SPM) analysis compared to 44 healthy subjects | Olfactory, amygdala, hippocampal, parahippocampal, cerebellar, and brainstem bulbo-protuberantial hypometabolism in all patients | Hypometabolism correlated with the number of symptoms and associated with hypo/anosmia, cognitive complaints, pain, and sleep disturbances |
| Guedj et al, 2020 France | Monocentric retrospective (case reports) | 2 | 54 and 62 y | 8 wk | Anosmia and memory disturbances; pain | Individual visual and semiquantitative SPM analysis compared to 23 and 24 healthy subjects | Case 1: olfactory hypometabolism; Case 2: olfactory, amygdala, hippocampal, parahippocampal, cingulate, rolandic, thalamic, hypothalamic, cerebellar, and brainstem bulbo-protuberantial hypometabolism | Analysis not feasible |
| Morbelli et al, 2022 Italy | Monocentric retrospective | 21 | 62 y (mean) | 14 wk | Hypo/anosmia (n = 21) | Group semiquantitative SPM analysis compared to 23 post-COVID subjects without hypo/anosmia | Fronto-temporal hypometabolism, particularly olfactory | Hypometabolism correlated with olfactory test (sniff test) |
| Sollini et al, 2021 Italy | Monocentric retrospective | 13 | 54 y (mean) | 132 d (mean) | Dyspnea (n = 9), fatigue (8), anosmia/ageusia (4), pain (5) | Group semiquantitative SPM analysis compared to 26 cancer control subjects | Olfactory, parahippocampal, and brainstem hypometabolism | Hypometabolism associated with fatigue and anosmia/ageusia |
| Dressing et al, 2022 Germany | Monocentric retrospective | 31 (14 with PET) | 54 y (mean) | 202 d (mean) | Cognitive disturbances (n = 31), fatigue (24) | Group semiquantitative SPM analysis compared to a control group of 45 subjects using a potentially biased activity normalization method [50] | No hypometabolism detected | |
| Morand et al, 2021 France | Monocentric retrospective (case reports) | 7 | 10–13 y | 5 mo (mean) | Fatigue (n = 5), dyspnea (4); pain (4); hypo/anosmia (3); hypo/ageusia (3); cognitive complaints (5); sleep disturbances (4) | Individual and group semiquantitative SPM analysis compared to 21 children with functional complaints before COVID and no neurologic diagnosis | Olfactory, amygdala, hippocampal, parahippocampal, cerebellar, and brainstem bulbo-protuberantial hypometabolism at the group level with partial individual recovery in 2 children reassessed at 6 and 12 mo postinfection | Analysis not feasible |
| Goehringer et al, 2023 France | Monocentric retrospective | 28 | 46 y (mean) | 16 mo (mean) | Cognitive disturbances (n = 28); headaches (13); fatigue (24); dyspnea (19); anosmia/ageusia (11); joint or muscle pain (15); digestive disturbances (7); cardiovascular symptoms (12); hyperventilation (15); dysautonomia (8); psychological disturbances (12) | Group semiquantitative SPM analysis compared to 28 healthy subjects | Fronto-temporal hypometabolism, particularly olfactory and internal temporal | Hypometabolism correlated with dyspnea and cognitive disturbances (MOCA) and associated with asthenia and language disturbances. Negative correlations also with the number of initial symptoms and duration of evolution |
| Verger et al, 2022 France | Multicentric (n = 3) retrospective | 143 | 47 y (mean) | 11 mo (mean) | Fatigue, pain, cognitive complaints, sleep disturbances, hypo/anosmia, hypo/ageusia, dysautonomia | Individual visual analysis based on olfactory, limbic/paralimbic brainstem, and cerebellar involvement | 26% of patients with severe hypometabolism in this network; 21% with moderate hypometabolism; 53% with normal metabolism | Data not available |
The cerebral anomalies observed in patients with long COVID have been correlated with various clinical symptoms. Neuropsychological assessments revealed cognitive deficits associated with cerebral glucose hypometabolism, particularly in regions critical for cognitive functions, further underscoring the impact of COVID-19 on brain health. For instance, brain hypometabolism, particularly in the olfactory gyrus and limbic/paralimbic regions, has been correlated with persistent neurocognitive symptoms such as memory impairment, attention deficits, and executive dysfunction. , Another study identified a diffuse pattern of hypometabolism in the frontal and temporal lobes, where the degree of hypometabolism was inversely correlated with the number and duration of symptoms, as also initially reported by the first French study, suggesting that the extent of metabolic dysfunction may directly reflect the severity and persistence of clinical manifestations. Additionally, olfactory dysfunction of long COVID has been correlated to metabolic signatures in brain regions crucial for olfactory processing, further linking these cerebral anomalies to specific sensory deficits. Interestingly, the metabolism of the frontal cluster that included the olfactory gyrus was decreased more starkly in patients treated with angiotensin-converting enzyme (ACE) drugs, and higher among patients who had used nasal decongestant spray, suggesting a possible role of ACE receptors as an olfactory gateway for this neurotropism.
Moreover, FDG-PET studies reveal a dynamic and evolving metabolic profile of COVID-19, reflecting the disease progression from the acute phase to more chronic stages. During the acute and subacute phases, patients may exhibit notable hypermetabolism in critical brain regions such as the limbic/paralimbic system, brainstem, and cerebellum, likely resulting from intense inflammatory responses triggered by the virus. , Alternatively, this hypermetabolism may occur as a compensatory response to localized hypometabolism or as part of the brain’s adaptation to ongoing inflammatory stress. Overtime, these metabolic patterns can shift, with some regions that initially displayed hypermetabolism potentially transitioning into hypometabolism as the disease progresses.
Encouragingly, there is evidence that some of these metabolic anomalies may be reversible. A longitudinal study tracking patients over 9 months revealed minor improvements in the pons and cerebellum, suggesting very partial reversibility of these metabolic abnormalities in patients with persistent symptoms. On the opposite, this recovery was often associated with improvements in cognitive function, highlighting the brain’s resilience and capacity for metabolic normalization overtime. As patients move from the acute to chronic stages, these metabolic anomalies may fluctuate, reflecting the brain’s ongoing response to the virus and the body’s inflammatory state. The observed partial recovery, particularly in regions that initially exhibited hypermetabolism, suggests that the long-term neurologic impacts of COVID-19 might be mitigated, leading to a gradual normalization of brain function.
Concerning pediatric patients, research has shown that the cerebral metabolic profile observed in long COVID is remarkably similar to those reported in adults. Specifically, regions such as the olfactory gyrus, and more broadly limbic/paralimbic areas, and the brainstem and cerebellum were consistently affected in both children and adults. These findings highlight that, despite differences in symptom presentation between age groups, the metabolic disruptions caused by COVID-19 affect similar brain regions in both children and adults.
Overall, FDG-PET studies have provided profound insights into the brain impact of long COVID in both adults and children as individual-level biomarker. The affected areas predominantly include a network of brain regions such as the olfactory bulbs, limbic and paralimbic structures (including the amygdala, hippocampus, insula, and cingulate cortex), brainstem, and cerebellum, which are critical for cognitive, sensory, autonomic functions, and emotional regulation. The metabolic anomalies observed, including both hypometabolism and occasionally hypermetabolism, are distinct from those seen in other neurologic or psychiatric disorders, suggesting a unique metabolic signature of COVID-19’s impact on the brain. The consistent findings across different populations and age groups highlight the pervasive nature of long COVID’s brain effects and support the hypothesis that the virus may use a “nose-to-brain” pathway. The dynamic nature of these metabolic changes, with evidence of potential reversibility, offers hope for recovery but also underscores the need for continued monitoring and intervention to mitigate long-term neurologic and psychiatric consequences.
Other Brain PET Targets
While 18F-FDG PET imaging has been widely utilized to investigate the brain impacts of long COVID, other radiotracers have been mostly used to explore brain inflammation and neurochemical changes associated with long COVID. One notable area of research involves the use of PET imaging to evaluate neuroinflammation. Radiotracers which binds to the TSPO associated with activated microglia, have been employed to assess inflammatory processes in the brain. Studies have indicated elevated TSPO levels in patients with long COVID, especially within the hippocampus, prefrontal and cingulate cortex, basal ganglia, and the pons, suggesting that neuroinflammation may play a significant role in the neurologic and psychiatric symptoms experienced by these individuals. , , , , This finding aligns with the hypothesis that COVID-19 can trigger an inflammatory response in the central nervous system.
Alternatively, research has also focused on the potential impact on neurotransmitter systems. , , PET studies examining dopamine and serotonin receptors have provided insights into the neurochemical reductions that may accompany long COVID, particularly in relation to mood disorders and cognitive impairment. Another promising avenue of research involves the use of radioligands targeting specific neuroreceptors or proteins. For instance, studies utilizing PET tracers are conducted to assess amyloid deposition in the brains of patients. These studies will aim to determine whether COVID-19 may accelerate neurodegenerative processes, particularly in individuals with preexisting vulnerabilities. Preliminary findings suggest that some patients exhibit increased amyloid burden, raising concerns about the potential long-term consequences of COVID-19.
Whole-Body PET Imaging
Recent studies utilizing whole-body imaging techniques, particularly total-body multiparametric PET, have also provided significant insights into the long-term effects of COVID-19. Authors revealed increased FDG uptake in various non-brain tissues from a case-control study, highlighting the systemic nature of long COVID. Specifically, they observed elevated FDG uptake in the lungs, bone marrow, large joints, and vessels, with a distinct pattern of mild-to-moderate uptake in these areas among patients with long COVID. These findings were confirmed in another study with increased FDG uptake in the lungs and bone marrow. Additionally, a retrospective study focusing on vascular hypermetabolism identified aortic FDG-PET hypermetabolism in 21% of the patients with long COVID. This vascular hypermetabolism, particularly in the thoracic aorta, was associated with symptoms such as persistent chest pain and dyspnea. The findings of these various studies suggest a persistent inflammatory response in multiple organs, further supporting the role of whole-body metabolic imaging in understanding long COVID.
Furthermore, using more innovative PET targets, a study revealed persistent tissue-based T-cell activation and viral RNA presence up to 2 years postinfection, highlighting the protracted nature of immune responses in patients with long COVID. This underscores the potential for ongoing viral activity and immune dysregulation as a basis for the chronic symptoms observed in long COVID. Finally, by utilizing dynamic PET with a CD8-targeted minibody to measure CD8+ T-cell distribution in vivo, significant immune activity was found months after infection, demonstrating the potential of dynamic PET for studying the total-body immune response in patients with long COVID.
Together, these findings reinforce the importance of advanced imaging modalities in elucidating the complex pathophysiology of long COVID, paving the way for more targeted therapeutic interventions.
MR neuroimaging in long coronavirus disease
Among other noninvasive imaging techniques, MR imaging has the ability of capturing multiple aspects of the central nervous system through multimodal acquisition, in possible combination with PET (PET-MR imaging). Whether by estimating the volume or alteration of anatomic structures (anatomic, morphometry), by quantifying white matter (WM) integrity (diffusion), or by investigating changes in brain activity (functional, connectivity), MR imaging also became a central part in the understanding of the neurologic impact of SARS-CoV-2. ,
Anatomic and Diffusion MR Imaging
One of the most impactful MR imaging studies provided compelling evidence of both structural and functional changes in the brain by comparing pre-COVID and post-COVID scans of 394 participants from the UK Biobank. They reported reduction in gray matter (GM) thickness and contrast in the orbitofrontal and parahippocampal area along with an overall reduction of brain size in patients with post-COVID pointing toward structural alteration of the limbic system.
Studies on diffusion imaging observed aberrant WM fractional anisotropy in the thalamus that correlated with fatigue complaints or decreased WM integrity in the brainstem (also associated with fatigue). Evidence of neuroinflammatory processes has been supported by increased free-water and mean diffusivity in the WM that could be seen as proxy for local immune response. Altogether, these findings suggest structural changes both in the GM and WM of limbic and subcortical area that may come from neuroinflammatory response to the disease. Hypotheses on the neuropathological aspect of the infection suggest systemic inflammatory processes or infiltration of the blood–brain barrier.
Functional MR Imaging
Many patients show clear cognitive deficits despite the absence of apparent structural brain lesions. fMR imaging, which captures blood oxygen levels reflecting neuronal activity, is crucial in analyzing brain functions in such cases. While fMR imaging has not been clinically validated, it has led to pivotal findings in the context of COVID-19. fMR imaging studies have reported altered patterns of functional connectivity 6 to 9 months after the initial infection, varying according to the severity of respiratory symptoms in the acute phase. These differences in connectivity partly explain cognitive deficits. Overall, reduced functional connectivity between cortical areas—particularly within the dorsal attention and somatosensory motor networks—was observed, while increased cortico-subcortical connectivity (eg, putamen, cerebellum) correlated with acute severity. Overall, the authors observed that functional connectivity was lower between cortical areas, particularly within the dorsal attention and somatosensory motor networks, while cortico-subcortical (putamen, cerebellum) connectivity increased, correlating with acute severity. Alterations in networks involved in consciousness and self-awareness have also been reported, independent of the severity of the acute infection. , For instance, increased functional connectivity in the default mode network (DMN) has been observed in COVID-19 patients with disorders of consciousness 3 to 6 months postinfection. Similarly, higher fractional rates of dynamic functional brain states were identified in COVID-19 patients with high rates of PTSD 6 months postinfection. A study on resting-state fMR imaging conducted 2 years after infection observed increased amplitude of low-frequency fluctuation (ALFF) in subcortical areas (left putamen, right pallidum) and decreased ALFF in temporal and parietal networks. Additionally, lower regional homogeneity in the precentral and postcentral gyri was correlated with increased mental fatigue and cognitive complaints.
Are There Distinct MR Imaging/Neuropsychological Profiles?
The heterogeneity in neuropsychological profiles among patients with COVID-19 has been highlighted, with at least 3 distinct clinical phenotypes of cognitive disorders identified. , The most discriminant factor among these phenotypes was self-awareness of cognitive impairment. Some patients exhibited a lack of awareness of their cognitive deficits despite objective memory impairments (anosognosia), while others reported significant complaints like fatigue but demonstrated only mild attentional and executive dysfunctions. , A third group had normal cognitive performance, but their complaints matched their subjective experiences. These findings suggest that alterations in functional connectivity, particularly in the DMN and networks related to consciousness and self-awareness, could underpin these diverse clinical phenotypes. A significant challenge for future studies is that patients diagnosed with post-COVID syndrome often correspond to only one clinical phenotype. Consequently, the phenotype characterized by memory impairments with a lack of awareness of their deficits may not be adequately represented in these cohorts due to the absence of self-reported complaints. This could lead to an incomplete understanding of the full spectrum of cognitive impairments associated with post-COVID syndrome. Future research must consider using relevant assessments and targeted recruitment strategies to ensure all phenotypes are adequately studied.
Future Directions for MR Imaging
In summary, both structural, anatomic, and fMR imaging alterations of the brain are coherent with neurologic outcome in the limbic, subcortical, and cerebellar area. Evidence of brain functional impairment in the sensory and DMNs has been put in relationship with cognitive disorders and points to a general alteration of the systems of memory, self-awareness, and emotion processing. Findings, modalities, and acquisition scheme have, however, been quite heterogeneous and have sometime failed to reach consensus. Nevertheless, further investigating multimodal neuroimaging markers after SARS-CoV-2 infection either with anatomic, diffusion, or functional imaging is key to uncover the wide range of long COVID trajectories and provide accurate policies to take care of patients.
Pathophysiological implications of neuroimaging PET findings and long-term prognosis
Understanding the mechanisms behind the brain metabolic PET dysfunction observed in patients with COVID-19 is critical for developing targeted treatments and interventions. One leading hypothesis focuses on the role of astrocytes, a type of glial cell that plays a crucial role in supporting neuronal and synaptic activity by regulating energy metabolism in the brain. Astrocytes are vital for maintaining glutamatergic neurotransmission, the primary excitatory signaling pathway in the brain. Researchers propose that SARS-CoV-2 infection, coupled with the associated neuroinflammatory response, may disrupt astrocyte function, leading to an imbalance in glutamate homeostasis. This dysregulation could underlie many of the cognitive impairments and other neurologic symptoms experienced by patients with long COVID. Moreover, the idea that therapies targeting astrocyte function and glutamatergic neurotransmission could alleviate some of the neurologic symptoms of long COVID has gained traction. Potential treatments include medications that enhance astrocytic glutamate uptake and agents that modulate N-Méthyl-D-Aspartate (NMDA) receptor activity, which is implicated in excitotoxicity.
Concerning the possible recovery and persistence of brain sequela, longitudinal studies have been particularly illuminating in offering insights into metabolic and structural brain changes. A notable study using FDG-PET followed 56 patients with long COVID over approximately 9 months, comparing their brain scans to those of 51 healthy individuals. The characteristic hypometabolic profile persisted in these patients with durable symptoms, with only minor improvements observed in certain areas, such as the pons and cerebellum, over the follow-up period. , , As previously mentioned, another MR imaging study reported global atrophy and reductions in GM volume in key regions like the orbitofrontal cortex and parahippocampal gyrus when compared to pre-pandemic scans. These structural alterations, combined with the persistent metabolic deficits observed in PET imaging, paint a concerning picture of the long-term neurologic impacts of COVID-19.
Beyond these imaging findings, emerging epidemiologic data have revealed the substantial cognitive toll of COVID-19, even in individuals who experienced only mild infections. One study involving nearly 113,000 people found that those who had been infected with SARS-CoV-2 exhibited significant deficits in memory and executive function tasks compared to uninfected individuals. The degree of cognitive impairment correlated with disease severity, with even mild COVID-19 associated with a measurable drop in intelligence quotient (IQ). Another study of over 100,000 Norwegians documented persistent memory impairments up to 3 years after a positive SARS-CoV-2 test. The cognitive deficits observed could have profound implications for educational attainment, workforce productivity, and the future burden of dementia and other neurodegenerative diseases. Finally, these concerns raise the possibility that COVID-19 could more directly influence neurodegenerative processes, leading to long-term cognitive decline in part of patients.
Current recommendations for fluorodeoxyglucose brain PET imaging, and perspectives
Given the growing recognition of COVID-19’s impact on brain health, the European Association of Nuclear Medicine (EANM) Neuroimaging Committee has provided guidance on the use and interpretation of 18F-FDG PET in the context of long COVID. The committee emphasizes that the characteristic hypometabolic pattern seen in patients with long COVID, involving the olfactory system, more broadly the limbic and paralimbic regions, the brainstem, and cerebellum, appears distinct from the metabolic profiles typically observed in other neurologic or psychiatric disorders.
However, the committee also cautions that nuclear medicine physicians must be vigilant in their interpretation, as subtle or isolated metabolic changes in certain brain regions (eg, the medial temporal lobe) should not automatically be attributed to long COVID. Preexisting neurologic or psychiatric conditions, as well as potential confounding factors like deconditioning, must be carefully considered. Additionally, the committee recommends that intensity normalization for PET quantification should preferentially use global cortical or maximal cortical voxel values rather than relying on regions like the pons or cerebellum, which may also be affected in long COVID. Pediatric cases require highly specialized interpretation, as it is crucial to account for age-related variations in brain metabolism that can significantly influence diagnostic accuracy.
As research continues to evolve, several key perspectives emerge regarding the role of brain PET imaging in this context. First, PET imaging can serve as a valuable diagnostic tool for identifying and characterizing the brain manifestations of long COVID. By providing insights into metabolic activity and inflammation, PET imaging can help differentiate between various neurologic conditions that may present similarly to long COVID symptoms, especially in older patients. This capability is crucial for establishing accurate diagnoses and tailoring appropriate treatment strategies for affected individuals.
Second, the integration of brain PET imaging with other imaging modalities, such as MR imaging, can enhance the understanding of the structural and functional changes occurring in the brain due to long COVID. Allowing researchers and clinicians to correlate metabolic alterations with structural abnormalities, this multimodal approach may lead to more precise assessments of the impact of long COVID-19 on brain function and facilitate the identification of potential biomarkers.
Moreover, brain PET imaging could play a pivotal role in monitoring the progression of long COVID and evaluating the effectiveness of therapeutic interventions. Longitudinal studies utilizing PET imaging can help track changes in brain metabolism overtime, providing insights into the reversibility of neurologic symptoms and the potential for recovery. This information is essential for developing targeted rehabilitation strategies and assessing their impact on brain health.
Additionally, the exploration of novel radiotracers beyond FDG may further enhance the utility of brain PET imaging in long COVID research. For instance, radiotracers targeting neuroinflammation, amyloid deposition, TAU phosphorylation, or specific neurotransmitter systems could provide deeper insights into the underlying pathophysiological mechanisms of long COVID. These advancements could lead to the identification of new therapeutic targets and inform the development of personalized treatment approaches.
Finally, as the understanding of long COVID continues to grow, brain PET imaging may contribute to the broader field of post-viral syndromes. Insights gained from long COVID research could inform the management of other post-viral conditions, enhancing the overall understanding of how viral infections impact brain health.
Addressing these challenges will require a multifaceted approach. First and foremost, continued research is essential to fully understand the long-term brain effects of COVID-19 and to develop effective treatments and interventions. This research should include large-scale longitudinal studies that follow COVID-19 survivors over many years to track the evolution of brain changes and cognitive function.
Summary
To conclude, the neurologic impacts of COVID-19 represent a significant and emerging public health challenge. From acute conditions like strokes to persistent symptoms such as cognitive decline, the effects of COVID-19 on the brain are diverse and complex. Neuroimaging studies, particularly those using FDG-PET, have revealed a characteristic pattern of hypometabolism associated with long COVID, offering crucial insights into the virus’s impact on the brain, on regions also showing structural alterations on MR imaging studies. Understanding the mechanisms behind these neurologic and psychiatric effects, including the potential role of astrocyte dysfunction and glutamate dysregulation, is vital for developing effective treatments. Long-term studies and continued research will be essential to fully elucidate the brain changes associated with COVID-19 and to develop therapeutic strategies. As the world continues to grapple with the ongoing effects of the pandemic, addressing the neurologic and psychiatric consequences of COVID-19 must be a priority. By investing in research, health care infrastructure, and global cooperation, the scientific and medical community can work to mitigate the long-term effects of COVID-19 on brain health.
Clinics care points
- •
The characteristic hypometabolic PET pattern seen in long COVID patients, involving the olfactory system, more broadly the limbic and paralimbic regions, the brainstem and cerebellum, appears distinct from the metabolic profiles typically observed in other neurological or psychiatric disorders.
- •
Nuclear medicine physicians must be vigilant in their interpretation, as subtle or isolated metabolic changes in certain brain regions (eg, the medial temporal lobe) should not automatically be attributed to long COVID.
- •
Pre-existing neurological or psychiatric conditions, as well as potential confounding factors like deconditioning or differential diagnosis, must be carefully considered.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree