Abstract
Wunderlich syndrome (WS), or spontaneous renal hemorrhage, is a rare and serious condition that demands prompt diagnosis and management. This report describes a 26-year-old female patient who experienced severe right-sided flank pain and hypovolemic symptoms during hemodialysis. The patient had several comorbidities, including poorly managed diabetes mellitus, diabetic nephropathy, and arterial hypertension. An active bleeding leading to a subcapsular renal hematoma was discovered by imaging. Using interventional radiology, immediate renal arterial embolization was part of the initial therapy. Recurrent bleeding required an emergency hemostatic nephrectomy despite temporary stabilization. This example emphasizes the importance of a hybrid management strategy that combines interventional radiology and surgical competence. It emphasizes how crucial a multidisciplinary team is to customizing interventions that strike a balance between the patient’s underlying chronic comorbidities and the urgent requirements of a life-threatening illness. This all-encompassing strategy produced a favorable result.
Introduction
Wunderlich syndrome (WS), characterized by spontaneous renal bleeding without trauma, is a rare and possibly fatal illness that necessitates immediate diagnosis and treatment. It was first described over 3 centuries ago and continues to be a diagnostic challenge due to its wide range of appearances.
The most common underlying causes include renal tumors, particularly angiomyolipomas and renal cell carcinoma, as well as vascular diseases like polyarteritis nodosa or arteriovenous malformations. This report examines the rare case of chronic hemodialysis as a probable cause of spontaneous subcapsular kidney hemorrhage in a 26-year-old patient with poorly managed diabetes. The case demonstrates the difficult balance of dealing with acute emergencies and chronic comorbidities through timely imaging, interventional radiology, and surgical therapy. The multidisciplinary approach emphasizes the need to combine new diagnostic technologies and therapy options to improve outcomes for this rare but crucial ailment.
Case report
A 26-year-old female patient with a medical history of diabetes mellitus treated with insulin was diagnosed during childhood following the onset of asthenia, weight loss, thirst, and constant polyuria. The patient has complications such as diabetic nephropathy and diabetic retinopathy, mainly due to poor treatment adherence. The patient’s condition progressed to end-stage renal disease, for which she needed hemodialysis 3 times per week. Additionally, she has been diagnosed with hypothyroidism treated with levothyroxine, and arterial hypertension.
The patient has no surgical history, and she has a mother treated for type 1 diabetes. During a hemodialysis session, the patient suddenly developed acute right-sided flank pain. The pain was severe and unresponsive to analgesics, necessitating an immediate referral to the emergency department.
She received a rapid initial clinical examination. The patient was afebrile and conscious, with a pale mucocutaneous complexion. Stable hemodynamically with a heart rate of 120 bpm, blood pressure at 90/60 mmHg, no peripheral hypoperfusion signs, and preserved diuresis. She was eupneic with an SpO 2 of 98% on ambient air and no respiratory distress. Right flank tenderness was noted without palpable masses.
The laboratory workup showed hemoglobin at 6.5 g/dL, sodium at 132 mmol/L, potassium at 4.5 mmol/L, urea at 1.46 g/L, and creatinine at 79.5 mg/L. The coagulopathy profile showed a platelet count of 325,000/µL, a prothrombin ratio of 74%, and a partial thromboplastin time of 1.11. As for arterial blood gas: pH: 7.38, PaCO₂: 32 mmHg, PaO₂: 103 mmHg, bicarbonate: 18.9 mmol/L, base excess (BE): −2 mmol/L. The urine cytobacteriological analysis was negative.
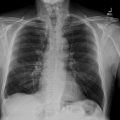
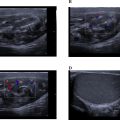
Following an early abdominal ultrasound that revealed a subcapsular right renal hyperechogenic mass, the patient underwent an emergency contrast-enhanced CT urogram, which revealed a nonruptured, nontumoral, hypodense right renal subcapsular collection that did not enhance after injection, measuring 100 × 30 mm, with no signs of tissue necrosis suggesting a subcapsular renal hematoma. An infiltration of the right psoas muscle was also observed ( Fig. 1 ).

The management plan includes the immediate installation of 2 peripheral venous lines, fluid resuscitation, serial arterial blood gas analysis, insulin delivery based on capillary glucose levels, and blood transfusions.
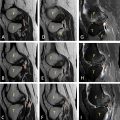
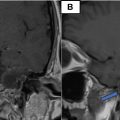
Following the diagnosis of the subcapsular renal hematoma with active bleeding, and given the relatively hemodynamic stability, a multidisciplinary decision was made for urgent management by the interventional radiology team ( Fig. 2 ).

After informed and written consent by the patient and explaining the procedure and potential risks. Using the Seldinger technique and under local anesthesia at the access site, a guide wire is inserted into the femoral artery under fluoroscopic guidance, and then a vascular sheath (5F) is placed in the common femoral artery to facilitate catheter insertion.
A curved angiographic catheter is advanced, and selective renal angiography is then performed to identify the target vessel; extravasation of the contrast agent was spotted in the lower sector. A coil was used to achieve successful embolization.
Postembolization angiography ensures complete occlusion of the targeted vessels and identifies any accessory arteries that may require additional embolization ( Fig. 2 ).
The puncture site is compressed with a sterile dressing, and the patient is placed on bed rest for 24 h. Ongoing care included monitoring renal function, electrolytes, and hemoglobin levels during hospitalization.
The patient had tachycardia and slight hypotension on the second day following the embolization. In light of the clinical situation, an urgent Uro-CT scan was done, which indicated a persistent hematoma and aggressive bleeding in the arterial phase.
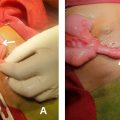
The patient was already on chronic hemodialysis; thus, the vital prognosis took precedence over the functional prognosis, and 1 kidney would suffice for effective diuresis. An emergency hemostatic nephrectomy was then performed. The patient was brought to the surgery room after being quickly resuscitated and hemodynamically stable. A subcostal incision was used to improve visibility of the renal pedicle.
After moving the colonic angle and opening the retroperitoneum, a significant renal gap was seen. The retroperitoneal hemorrhage was modest, most likely due to blood being retained under the renal capsule and as a result of the prior embolization.
A renal pedicle ligation was performed, followed by nephrectomy and placement of a drainage tube (Redon). The postoperative course was characterized by good improvement, hemodynamic stability, cessation of deglobulization, preserved diuresis, and a drainage tube that only returned a few traces of sero-hematologic fluid. The patient was discharged on the fifth day postoperatively after the drainage tube was removed on day 2 and given an oral antibiotic prescription based on her renal function ( Fig. 3 ).