Table 19.1 Classification of Endoleaks | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||
Management of Stent-Graft Endoleaks
Management of Stent-Graft Endoleaks
Nathaniel C. Swinburne
Robert A. Lookstein
Endovascular Aortic Aneurysm Repair and Endoleaks
1. Certain anatomic considerations made before endovascular aortic aneurysm repair (EVAR) predispose to specific types of complications, including endoleaks (1). These include proximal/distal attachment sites, angulation, and tortuosity of the aorta, and the presence of calcification and occlusive disease in the access arteries.
3. With the more frequent use of EVAR, the occurrence of complications has risen. The most common complication is endoleak (25% to 35% of patients), which may progress to aneurysm expansion and rupture (3).
4. An endoleak is defined as persistent perfusion of the aneurysm sac outside of the stent graft.
5. A primary endoleak occurs during the first 30 days after stent-graft placement. A secondary endoleak occurs after the first 30 days after stent-graft placement following at least one negative cross-sectional study (1).
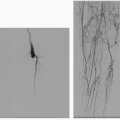
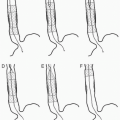
6. There are five types of endoleaks, which are defined by the etiology of blood flow into the aneurysm sac (4).
Endoleak Classification (see Table 19.1)
1. Type I endoleak
a. Source of blood flow is a stent-graft attachment site (either proximal attachment site [type IA], distal attachment site [type IB], or from an incompletely occluded, contralateral common iliac artery in aortouniiliac stent-grafts [type IC]) (1,4).
b. Type I endoleaks occur more commonly in the thoracic aorta and in aortas where the attachment sites are complicated by thrombus, short and/or angulated necks, and dilated, irregular iliac arteries (1,4).
c. Type I endoleaks can be seen immediately after deployment as a result of incomplete expansion of the stent-graft, aortic tortuosity, or steep aortic angulation (5).
d. Late development of type I endoleaks may be related to changes in the configuration of the aorta as the aneurysm sac shrinks (4).
2. Type II endoleak
a. Source of blood flow is the patent aortic branch vessels (most commonly the inferior mesenteric artery [IMA] and lumbar arteries), with retrograde flow into the aneurysm sac (5).
b. Other less common sources include the hypogastric, median sacral, accessory renal, and gonadal arteries.
c. Type II endoleaks are further classified into IIA, in which a single vessel is involved, and type IIB, in which two or more vessels are involved (4).
3. Type III endoleak
a. Source of blood flow is through a structural defect in the stent graft including junctional separations within modular devices (type IIIA), or stent-graft fractures or fabric holes (type IIIB) (1).
b. Type III endoleaks are likely caused by stresses from arterial pulsations or stresses resulting from aneurysm sac shrinkage.
4. Type IV endoleak
a. Source of blood flow is through porosity in the stent graft and manifests as a “blush” in the immediate postimplantation angiogram in patients who are fully anticoagulated (1).
b. Type IV endoleaks are a diagnosis of exclusion as other types of endoleaks can mimic type IV endoleaks.
c. Type IV endoleaks are rarely seen now due to improved stent-graft construction.
5. Type V endoleak
a. Continued aneurysm expansion in the absence of a confirmed endoleak, also referred to as endotension
Post-EVAR Imaging Modalities
Lifelong imaging surveillance is necessary after EVAR. An ideal protocol for imaging follow-up has not been established.
1. Radiography
a. Useful to monitor for stent migration, detect kinks in abdominal stent grafts, and evaluate conformation of thoracic stent grafts
b. Typical projections include anteroposterior (AP) and lateral (for migration and component separation) and oblique (to improve sensitivity for detecting stent-graft fractures).