Masses that May Mimic Soft Tissue Tumors
There are a large number of tumor-like lesions of soft tissue. Many of these have a clinical presentation simulating that of a soft tissue neoplasm. The lesions presented here are not an all-inclusive, comprehensive review of tumorlike masses, but merely our experience with lesions that may clinically present as musculoskeletal tumors. For organizational purposes, we have classified these into broad categories: mass-like lesions, crystal deposition diseases, traumatic lesions, and inflammatory masses. Lesions that may mimic soft tissue tumors that are typically located in the skin and superficial tissues are covered in Chapter 12.
MASS-LIKE LESIONS
There are a number of mass-like lesions that may simulate a soft tissue tumor. Some of these have very characteristic imaging appearances and occur in specific clinical circumstances. The diagnosis in such cases is usually definitive; unfortunately, the diagnosis in others can be far more challenging.
Amyloid
Amyloid deposition can occur throughout the musculoskeletal system, including bone, disc space, and soft tissues. Involvement in the soft tissues occurs primarily in the tenosynovium, capsule, ligaments, and tendons. Although it is convenient to think of amyloidosis as a specific disease process, it represents a heterogeneous group of disorders characterized by the extracellular deposition of distinctive insoluble protein fibrils. As a result of the unique spatial geometry, amyloid produces a characteristic apple green birefringence under polarized light, after staining with Congo red (1, 2).
Currently, the classification of amyloidosis is based on the specific type of protein fibril involved in the amyloid deposits, with at least 25 different types of protein fibrils identified (2, 3, 4). The vast majority of cases of primary amyloidosis may be defined as amyloid immunoglobulin light chain disease, amyloid light chain, or AL type, whereas amyloid associated with chronic infection, inflammation, or nonimmunocyte neoplasia is designated AA type (3, 5). Less common types are associated with chronic dialysis (β-2 microglobulin or Aβ2m), familial amyloid polyneuropathies and senile systemic amyloidosis (transthyretin amyloidosis or ATTR) (1, 3, 6). The type of amyloid immunoglobulin can have important clinical implications, since up to 80% of patients with AL-type amyloidoma in the abdomen or mediastinum have been reported to develop a lymphoplasmacellular malignant neoplasm (1). This classification system has generally
replaced the clinically based system in which amyloid was most often classified as primary (without coexistent or antecedent disease), secondary (associated with various chronic diseases), or heredofamilial (specific affected families), although other rare types are described (7).
replaced the clinically based system in which amyloid was most often classified as primary (without coexistent or antecedent disease), secondary (associated with various chronic diseases), or heredofamilial (specific affected families), although other rare types are described (7).
KEY CONCEPTS
The term amyloidosis is used for a heterogeneous group of disorders characterized by the extracellular deposition of unique protein fibrils.
Currently, the classification of amyloidosis is based on the specific type of protein fibril involved in the amyloid deposits.
Amyloid deposition in the soft tissues is most common in association with chronic inflammatory diseases and multiple myeloma.
Amyloid is seen most frequently with chronic renal failure in patients undergoing dialysis.
CT and MR imaging of tenosynovial amyloid deposition is seen as capsular thickening around a joint.
Extrinsic erosion of bone is not infrequent.
MR imaging signal intensity is similar to that of muscle on T1-weighted images and shows low-to-intermediate signal intensity on T2-weighted MR pulse sequences.
Amyloid deposition in the soft tissues is most common in association with chronic inflammatory diseases and multiple myeloma (5% to 10% of patients) (7), and seen most frequently with chronic renal failure, particularly, although not exclusively, in patients undergoing hemodialysis. The prevalence of Aβ2m amyloidosis (dialysis-associated amyloidosis) increases with the length of time on dialysis, reaching 80% to 100% after 20 years (8). Tenosynovial deposition of amyloid is usually in the shoulder and hip. Indeed, Camacho et al. showed an incidence of 71% and 35%, respectively, in these two joints in a group of 88 patients (9). Involvement is typically polyarticular. Deposition in the carpal tunnel, with median nerve compression, occurs in 2% to 31% of patients with chronic renal failure (7). Unlike idiopathic carpal tunnel syndrome, which shows predominance in women and in the right hand, no such predilection is seen in patients with amyloid deposition.
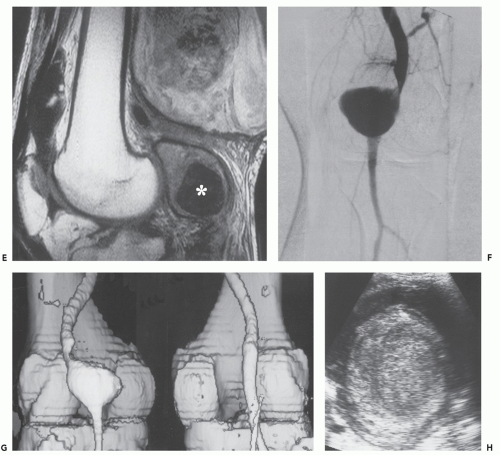
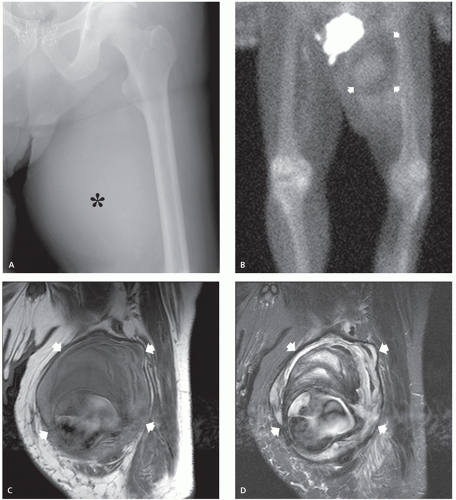
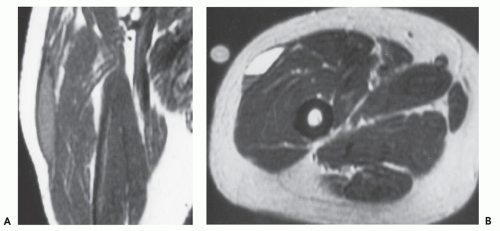
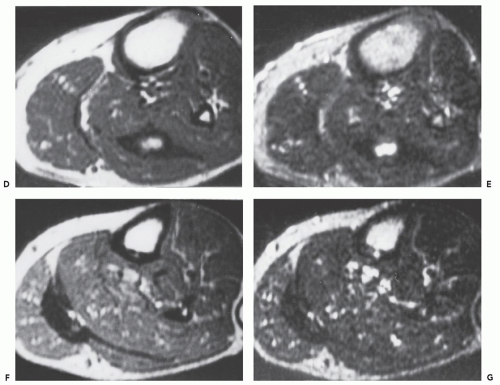
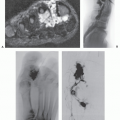
Computed tomography (CT) and magnetic resonance (MR) imaging of tenosynovial deposition of amyloid is seen as capsular thickening around a joint. In locations such as the carpal tunnel, it is often prominent and extensive (10, 11, 12, 13). Extrinsic erosion of bone is not infrequent, with punched-out lesions apparent on radiographs, particularly in the hips and shoulders. CT shows the thickening to be of soft tissue attenuation, whereas the signal intensity is similar to that of muscle on T1-weighted MR images. However, the most characteristic imaging appearance is the low-to-intermediate signal intensity on T2-weighted MR pulse sequences (Fig. 13.1) (8, 14). This appears to reflect the collagen-like pathology of amyloid. Small focal areas of fluid with high signal on fluid-sensitive MR images are also frequent. Amyloid deposition within a joint can simulate pigmented villonodular synovitis, although the polyarticular involvement typifies the former disease as opposed to monoarticular changes in the latter. In our experience, enhancement of amyloid disposition can be seen following intravenous contrast injection on MR imaging (Fig. 13.2).
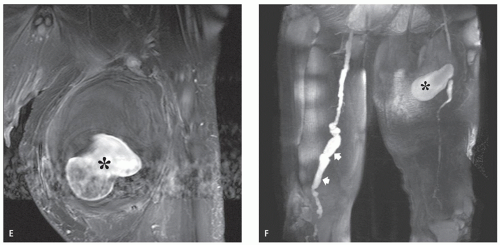
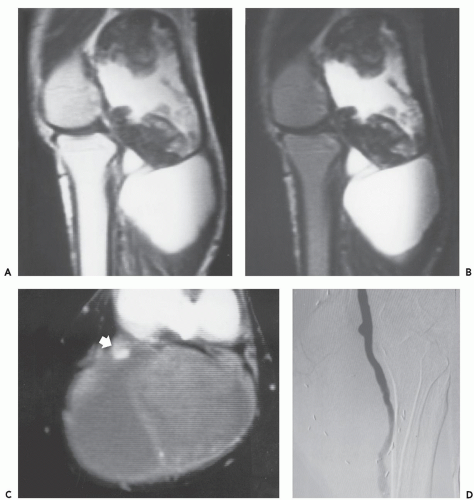
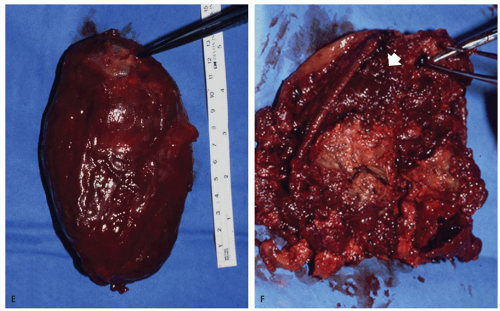
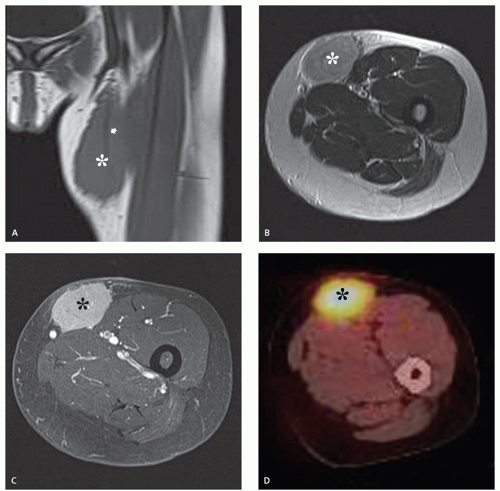
The least common presentation of amyloid deposition is as a discrete mass called amyloidoma or amyloid tumor, and is the form most likely to be confused with a soft tissue neoplasm (1, 3, 6). Maheshwari et al. (15) reported an amyloidoma in the thigh and reviewed the literature in 2009, noting a total of 21 documented cases (3, 6, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32). Interestingly, although common amyloidogenic conditions such as rheumatoid arthritis (AA type) and long-term hemodialysis (β-2 microglobulin type) predispose one to systemic amyloidosis, a focal amyloidoma in these patients is still rare.
The subgroup of extremity amyloidomas seems to be distinct from other soft tissue amyloidomas. Most of them have been reported in lower limbs (18 of 21, 86%)
and one case involved both upper and lower extremities. Of the 17 specified cases, AL type was reported in 7 cases (41%), AA type in 6 cases (35%), and β-2 microglobulintype in 4 cases (24%). Multiple sites were reported in all cases with β-2 microglobulin-type amyloidoma. Marginal surgical excision has been adequate for all amyloidomas, even for recurrence (23). Although long-term follow-up is not available, most of the cases of extremity amyloidomas have not developed systemic disease, lympho proliferative disease, or myeloma (22). Only three patients have been subsequently diagnosed with multiple myeloma (17, 20) or lymphoblastic dyscrasia (19).
and one case involved both upper and lower extremities. Of the 17 specified cases, AL type was reported in 7 cases (41%), AA type in 6 cases (35%), and β-2 microglobulintype in 4 cases (24%). Multiple sites were reported in all cases with β-2 microglobulin-type amyloidoma. Marginal surgical excision has been adequate for all amyloidomas, even for recurrence (23). Although long-term follow-up is not available, most of the cases of extremity amyloidomas have not developed systemic disease, lympho proliferative disease, or myeloma (22). Only three patients have been subsequently diagnosed with multiple myeloma (17, 20) or lymphoblastic dyscrasia (19).
There is scant literature documenting the MR imaging features of musculoskeletal amyloidoma, with reports limited to individual cases or limited case series, many with incomplete imaging details (11, 16, 17, 21, 33, 34, 35, 36, 37). Despite these limitations, MR imaging will generally reveal a heterogeneous focal mass on both T1-weighted and T2-weighted images (see Fig. 13.2), showing variable enhancement. However, some data can be extrapolated from amyloidosis reported in other systems (34, 35, 36, 37). The variable MR appearance may reflect the different immunocytochemical features of the lesion composition (2, 3, 4, 16, 17). Similarly, rapid chemical exchange
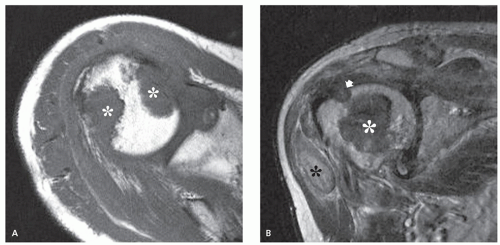
and spin-spin interactions may occur between the amyloid protein and adjacent water molecules, which may affect the signal intensity on MR imaging (36). Also, the signal may be affected by the differential amount of collagen, calcification, necrosis, vessels, and the type of amyloid fibril (36). Considerable variation exists, however, high signal intensity has also been reported to be attributable to the hemorrhagic or proteinaceous nature of amyloid deposition (16, 17, 21, 37). An amyloidoma may show a signal intensity similar to that of fat on T1-weighted images (Fig. 13.3), likely reflecting the extensive eosinophilic amorphous deposits within the lesion (21).
and spin-spin interactions may occur between the amyloid protein and adjacent water molecules, which may affect the signal intensity on MR imaging (36). Also, the signal may be affected by the differential amount of collagen, calcification, necrosis, vessels, and the type of amyloid fibril (36). Considerable variation exists, however, high signal intensity has also been reported to be attributable to the hemorrhagic or proteinaceous nature of amyloid deposition (16, 17, 21, 37). An amyloidoma may show a signal intensity similar to that of fat on T1-weighted images (Fig. 13.3), likely reflecting the extensive eosinophilic amorphous deposits within the lesion (21).
Aneurysm and Pseudoaneurysm
A peripheral aneurysm, especially when large, may mimic a soft tissue tumor (38, 39). Defined as a dilatation of the wall of a vessel, an aneurysm may be true or false. A true aneurysm contains all three layers of the arterial wall (intima, media, and adventitia), whereas a false or pseudoaneurysm contains only adventitia, and is often considered to be analogous to an intramural hematoma. In pseudoaneurysm, the adventitia and perivascular connective tissue remain intact and act to contain the vascular contents, preventing vascular rupture (40).
The popliteal artery is the most frequently affected by peripheral aneurysm (41, 42). Typically arteriosclerotic, up to 75% are bilateral, and 45% are associated with another aneurysm, especially of the abdominal aorta (41).
Patients are generally older men, in the fifth and sixth decades (41, 42). As many as half of the patients may be asymptomatic. When symptoms are present, they may suggest vascular occlusive disease, including claudication, rest pain, ulceration, and venous obstruction (41, 42, 43). Lesions are usually smaller than 5 cm, although aneurysms up to 22 cm have been reported (41, 43). Aneurysms may be complicated by extensive arteriosclerotic disease and internal thrombus.
KEY CONCEPTS
A true aneurysm contains all three layers of the arterial wall.
A false or pseudoaneurysm wall contains only adventitia.
The popliteal artery is the vessel most frequently affected by peripheral (typically arteriosclerotic) aneurysm.
Pseudoaneurysms are usually the result of injury to the arterial wall.
MR imaging in uncomplicated aneurysm cases reveals dilatation of the vessel, with a persistent flow signal void.
Pseudoaneurysms typically show a more complex signal intensity than aneurysms, with areas of increased and decreased signal intensity.
Distinction between an aneurysm with extensive thrombus and a pseudoaneurysm may be difficult.
Pseudoaneurysms are usually the result of injury to the arterial wall, with extravasation of blood and hematoma formation. The hematoma may compress and seal the injury (44). Lesions may remain stable for years, and symptoms may relate to the overall size of the lesion (44). Peripheral embolization is unusual (44). The diagnosis may be difficult in patients without a history of previous surgery, invasive procedure, arterial catheterization, or trauma (45, 46, 47, 48, 49, 50).
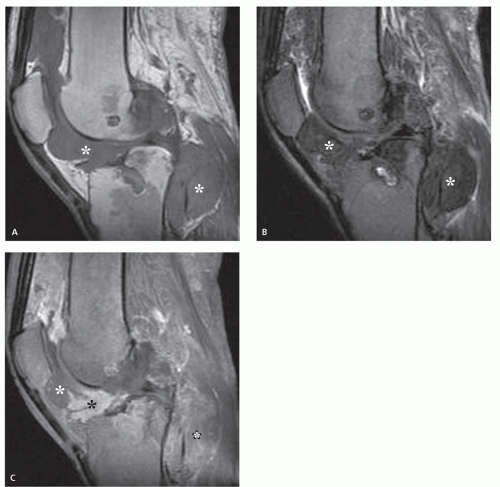
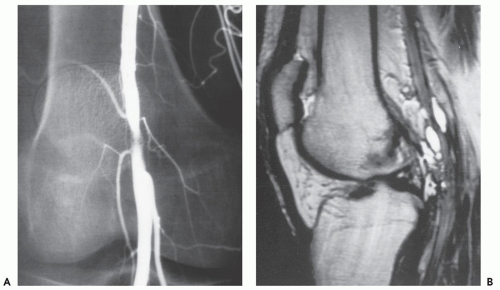
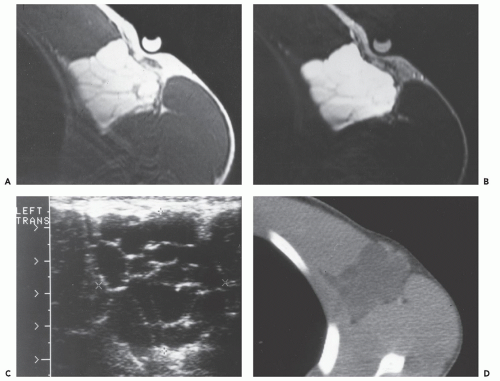
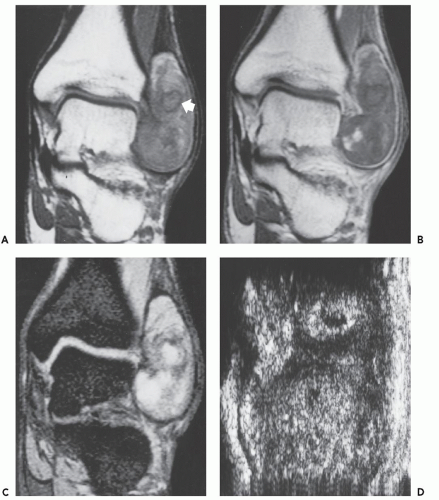
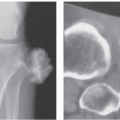
Radiographs reveal a nonspecific soft tissue mass. Curvilinear calcifications may be present, although the frequency of calcification is unknown (41), it is likely uncommon. MR imaging in uncomplicated cases reveals dilatation of the vessel, with a persistent flow signal void. Frequently, MR imaging demonstrates a complex mass: signal intensities vary with the size and morphology of the aneurysm and the relative amounts of residual lumen, turbulent flow, atheromatous plaque, fibrosis, subacute blood, and hemosiderin-laden tissue. Lesions are typically well-defined, with a round-to-elliptical shape. Infiltration of tissue planes beyond the aneurysm may be seen with leaking aneurysms (42). On conventional spin-echo MR imaging, aneurysms show a variable signal intensity on the basis of blood flow, showing flow void in areas of rapidly flowing blood and increased signal in areas of slowly flowing blood (Fig. 13.4). Pseudoaneurysms are usually elliptical soft tissue masses adjacent to the involved vessel. Pseudoaneurysms typically show a more complex signal intensity than aneurysms, with areas of increased and decreased signal intensity on all pulse sequences, corresponding to subacute blood and hemosiderin-laden tissue (Figs. 13.4 and 13.5). Gradient-echo images may be very useful in identifying hemosiderin-laden tissue. A flow void from the native vessel may be seen within or at the periphery of the mass. It has been our experience that the imaging distinction between an aneurysm with extensive thrombus and a pseudoaneurysm may be difficult.
CT scanning of an aneurysm shows a well-defined, homogeneous, low-attenuation mass (see Figs. 13.4 and 13.6) (51). Curvilinear calcification may be identified in the vessel wall. Subacute hemorrhage in pseudoaneurysm reveals decreased attenuation, typically at the periphery of the lesion, a distribution opposite to that of the central necrosis and hemorrhage seen in high-grade sarcomas (see Fig. 13.4). Ultrasound is especially useful for small lesions showing the association with the native vessel, saccular configuration of the lesion, and internal turbulent blood flow (52).
An aneurysm should be considered in the differential diagnosis when a mass is identified in the popliteal fossa region (or other area adjacent to vessels) and imaging shows a complex structure, especially with a lamellated appearance. Bilotta et al. (42) note that complete encasement (i.e., the vessel in the center of a soft tissue mass) or
obliteration of the popliteal vessels by a tumor is rare, and this finding should suggest an aneurysm.
obliteration of the popliteal vessels by a tumor is rare, and this finding should suggest an aneurysm.
Castleman Disease
Castleman disease is a rare lymphoproliferative disorder also known as angiofollicular hyperplasia, angiomatoid lymphoid hamartoma, giant lymph node hyperplasia, and follicular lymphoreticuloma (53, 54). It was originally described by Castleman et al. in 1956 (55), who reported 13 patients with enlarged mediastinal lymph nodes resembling thymic tumors radiologically, grossly, and even microscopically. Castleman disease remains one of the more common causes of nonneoplastic
lymphadenopathy and represents a form of polyclonal B-lymphocyte proliferation (56, 57).
lymphadenopathy and represents a form of polyclonal B-lymphocyte proliferation (56, 57).
There are two histologic variations: the hyaline vascular variant accounts for approximately 90% of cases, and the plasma cell variant accounts for the remaining 10% (53, 58, 59). The former consists of small lymphoreticular follicles distributed in a hypervascular, hyalinized stroma, whereas the latter consists of larger lymphoreticular nodules, separated by sheets of plasma cells, in a less vascular stroma (53, 60).
There are also two clinico radiographic subtypes: unicentric and multicentric. The former typically affects young patients, is usually localized to the mediastinum in a single node or nodal group, and presents with clinical symptoms related to lymphadenopathy (61, 62). The latter is associated with both human immunodeficiency virus (HIV) and human herpes virus-8 (HHV-8), is typically found in immunocompromised patients, and is associated with systemic symptoms (56, 61). Approximately 15% of patients with multicentric disease will have POEMS (polyneuropathy,
organomegaly, endocrinopathy, monoclonal gammopathy, skin changes) syndrome (61). POEMS syndrome may also be seen in patients with unicentric disease (62).
organomegaly, endocrinopathy, monoclonal gammopathy, skin changes) syndrome (61). POEMS syndrome may also be seen in patients with unicentric disease (62).
KEY CONCEPTS
Castleman disease is a rare lymphoproliferative disorder also known as angiofollicular hyperplasia.
It is classified on its histology as:
Hyaline vascular variant, which accounts for approximately 90% of cases.
Plasma cell variant, which accounts for the remaining 10% of cases.
The hyaline vascular form of Castleman disease:
Is unicentric about 90% of the time.
Typically presents with a mass localized to the mediastinum in a single node or nodal group.
Has clinical symptoms related to lymphadenopathy.
Plasma cell variant of Castleman disease:
Typically presents as multicentric disease.
Is frequently associated with constitutional symptoms, such as fever, sweating, and fatigue.
Is usually associated with both HIV and HHV-8 and is typically found in immunocompromised patients.
CT scanning in patients with typical, unicentric thoracic disease usually shows a rounded, solitary mass.
Prominent homogeneous contrast enhancement is characteristically seen on CT.
CT will show variable calcification.
MR imaging may demonstrate prominent vascularity, but is otherwise nonspecific.
Both men and women are affected about equally (54, 59). Patients range in age from the first through the sixth decade (54). The hyaline vascular form of Castleman disease (about 90% of cases) is unicentric 90% of the time (56). Approximately 70% of patients with the hyaline vascular type are younger than 30 years (59). Patients typically present with an asymptomatic, solitary mediastinal mass. However, symptoms may occur if the mass compresses adjacent structures. The mediastinum accounts for 40% to 70% of cases (53). Approximately 30% of cases are extrathoracic, and an estimated 10% to 40% are cervical (59, 61). Cervical lesions tend to occur laterally and may simulate lymphadenopathy (53). Other sites include the abdomen, pelvis, axilla, retroperitoneum, and mesentery (53, 61). Rarely, multicentric forms will be of the hyaline vascular type (56).
The plasma cell form of Castleman disease is most commonly multicentric, presenting as unicentric disease in 9% to 24% of cases (61, 62). Constitutional symptoms, such as fever, sweating, and fatigue, may be seen in approximately half of patients with the plasma cell variant (58, 61). There is also an association with hypergammaglobulinemia and hypoalbuminemia (58, 61).
There have been numerous reports of Castleman disease with systemic manifestations similar to those of a multicentric lymphoproliferative disorder (62, 63). This is seen with both histologic subtypes, but is more common in the plasma cell variant (62). These cases are characterized by an aggressive clinical course, multifocal lymphadenopathy, hepatosplenomegaly, and abnormal liver and renal function. There is an association with malignancy, usually lymphoma, although other malignancies have been reported (62). This phenomenon has led to a distinction between the aggressive multicentric form, which may be fatal, and the localized form, which may be an incidental finding (63). The multicentric form is usually seen in older men, with a mean age of 57 years (63).
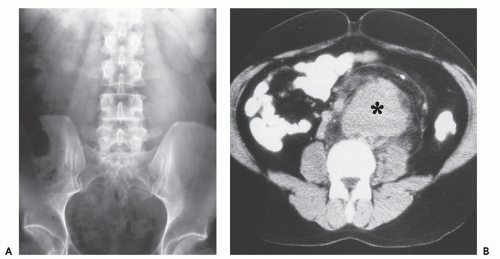
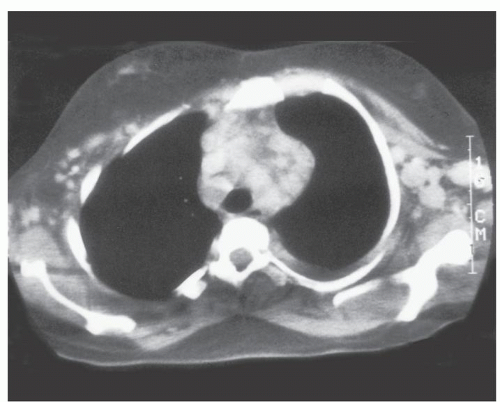
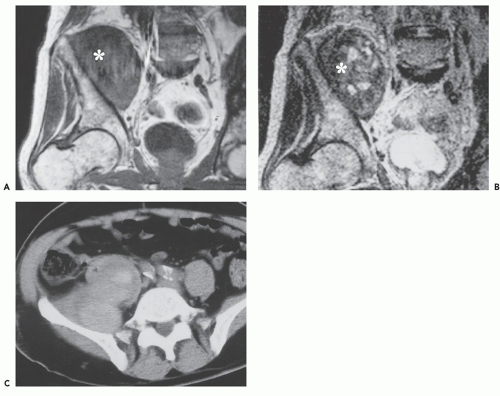
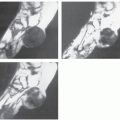
CT scanning in patients with typical thoracic disease usually shows rounded, solitary mediastinal or hilar mass (64). Pleural disease may present as a well-defined, interlobular mass or as a large pleural effusion (64). Following contrast administration, CT demonstrates homogeneous, intense, contrast enhancement or multicentric ring enhancement (53, 56, 59, 60, 63). In the abdomen and pelvis, a single, well-defined enhancing mass is also most frequently seen (65). Although the histologic basis for the enhancement pattern is unknown (Fig. 13.7), the pattern of enhancement is considered to be characteristic (64, 66). Lesions larger than 5 cm may show heterogeneous enhancement, with areas of decreased attenuation, caused by necrosis and degeneration (64, 65). Calcification within the lesion is noted (67, 68), and has been variably described as punctate, coarse, peripheral, and/or arborizing (61). Dense calcification is reported more frequently in retroperitoneal lesions (63). Calcification is seen in approximately one-third to one-half of abdominal and pelvic lesions and is described as having an arborizing pattern that appears to radiate from the center of the mass (65, 69, 70). The plasma cell variant characteristically shows multifocal lymphadenopathy, as well as hepatosplenomegaly, and is indistinguishable from adenopathy from other causes (Fig. 13.8).
Limited experience with MR imaging shows the lesion to have a nonspecific appearance. Most lesions are isointense or slightly hyperintense to skeletal muscle on T1-weighted images and heterogeneous, with variable, intermediate to mildly hyperintense signal intensity on T2-weighted images, and high signal intensity on fat-suppressed, fluid-sensitive images (56, 61, 64, 71, 72, 73). Prominent enhancement is typically seen and flow voids may be identified, reflecting feeding vessels and tumor vascularity (Fig. 13.9) (56). Central linear hypodensities have been reported, thought to represent lamellar fibrosis (72). Although lesions are usually hyperintense on fluid-sensitive sequences, intermediate signal intensity may be seen (73, 74).
At angiography, the mass is hypervascular, with large feeding vessels and a capillary blush (73). Sonography reveals a hypoechoic mass with prominent flow on power Doppler evaluation (64, 70).
Cystic Adventitial Disease
Cystic adventitial disease is a rare cause of lower extremity claudication, resulting from the formation of cystic collections of mucinous material within the adventitia of the popliteal artery, subsequently narrowing the arterial lumen (75, 76, 77). It is included here because it may appear as a soft tissue mass on MR imaging (75). Physical examination may demonstrate a soft tissue mass, and in the setting of a younger patient with no evidence of arteriosclerosis, clinicians often focus on the soft tissue mass, questioning a soft tissue sarcoma or a popliteal cyst, rather than a vascular cause. The basis of cystic adventitial disease is unknown, but possible causes are repetitive adventitial trauma, myxoid degeneration, and inclusion of mucus-secreting cell rests in the adventitia (76, 77). Currently, the most accepted hypothesis is that
cystic adventitial disease is an embryologic abnormality in which undifferentiated mesenchymal cells, which will eventually develop mucoid cysts, are incorporated into the vessel wall during vessel development (78).
cystic adventitial disease is an embryologic abnormality in which undifferentiated mesenchymal cells, which will eventually develop mucoid cysts, are incorporated into the vessel wall during vessel development (78).
KEY CONCEPTS
Cystic adventitial disease results from the formation of cystic collections of mucinous material within the arterial adventitia.
Any peripheral artery can be affected; however, it has a striking proclivity for the popliteal artery.
MR imaging demonstrates aggregates of multiple, small, round or oval masses originating in the wall of the involved peripheral artery.
Individual intramural masses show homogenous low signal intensity on T1-weighted images and high signal intensity on T2-weighted or fluid-sensitive sequences, reflecting the myxoid character of the lesion.
Angiography demonstrates the extrinsic, intramural nature of the stenosis.
Any peripheral artery can be affected; however, adventitial cystic disease has a striking proclivity for the popliteal artery. Cases have also been identified in the external iliac, femoral, radial, brachial, and ulnar arteries, as well as the external iliac, femoral, and saphenous veins (79, 80, 81). Bilateral involvement in the popliteal artery has been reported, but is exceedingly rare (82).
The disorder typically afflicts young to middle-aged men (male to female 5:1) in the third through fifth decades, without evidence of atherosclerosis or other systemic vascular disease (77, 79, 83, 84). Affected patients typically present with sudden onset of rapidly progressive calf claudication and lower extremity pain.
Lesions are typically small and unilateral, involving the adventitia (76). A long-standing lesion may become large enough to present as a soft tissue mass. Cystic adventitial disease occasionally extends into the media or produces popliteal artery thrombosis (76). The cystic lesions contain glycoproteins, hyaluronic acid, and hydroxyproline, and may rarely rupture spontaneously (76). Surgery is usually curative and popliteal involvement has been traditionally treated by resection of the narrowed arterial segment or bypass graft (79). Percutaneous ultrasoundguided cyst aspiration can be an effective alternative to surgery in patients who do not have thrombotic occlusion (76, 85). Limited experiences with angioplasty and stenting have been disappointing, attributed to the extraluminal nature of the disease (79).
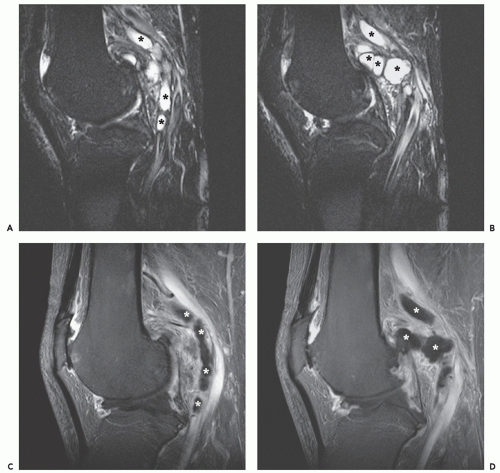
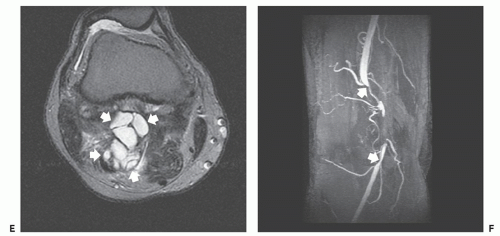
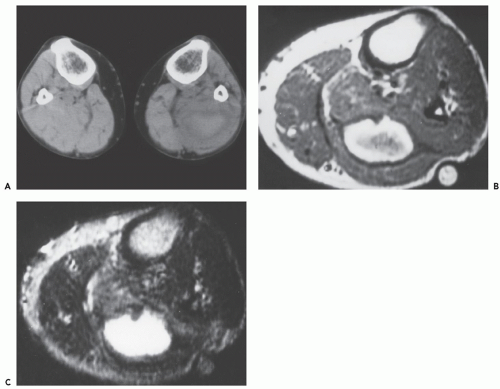
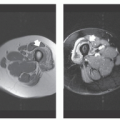
MR imaging demonstrates aggregates of multiple, small, round or oval masses, originating in the wall of the involved peripheral artery, with larger lesions having a multilobulated appearance. The individual intramural masses demonstrate homogenous low signal intensity on T1-weighted images and high signal intensity on T2-weighted or fluid-sensitive sequences, consistent with a cyst-like character. Following gadolinium administration, lesions typically do not enhance; however, mild peripheral enhancement may be seen (84, 86). The multilobulated cystic masses compromise the arterial lumen to varying degrees, which range from minimal eccentric indentation, to crescentic stenosis, to complete occlusion.
MR and conventional catheter angiography demonstrate the degree of luminal compromise and the extrinsic intramural nature of the stenosis. Stenosis is usually focal and relatively smoothly tapered, in the popliteal artery, and at the level of the femoral condyles (Figs. 13.10 and 13.11) (76). The stenosis may show an hourglass, curvilinear, or spiral configuration without post-stenotic dilation, or there may be complete occlusion (83). The stenosis may extend for several centimeters. The lesion shows a decreased attenuation on CT scanning, with rim enhancement following contrast administration (77, 83).
Sonography demonstrates multilobulated collections of small, rounded or oval, anechoic or hypoechoic masses arising in the walls of the affected arteries (79). Crescentic indentation upon the anechoic lumina of the vessels is seen with eccentric narrowing or occlusion by the cystic masses. This feature is also well-seen on angiographic studies (see Fig. 13.11). Low-level echoes may be seen within the masses, indicative of debris within the contents of the cysts (84).
Hematoma
Hematomas occurring in the extremities or trunk may clinically present as soft tissue masses, and must be
distinguished from tumors. Complicating this differentiation is the occurrence of hemorrhage into preexisting neoplasms.
distinguished from tumors. Complicating this differentiation is the occurrence of hemorrhage into preexisting neoplasms.
MR imaging is well-suited for the assessment of hematomas. The presence of hemoglobin and its degradation products, which are the major constituents of hemorrhagic collections, influence the MR imaging appearance of hematomas through alterations in T1 and T2 relaxation times, allowing for diagnosis. Because the status of hemoglobin within a hematoma is very dependent on the acute or chronic nature of the hemorrhagic collection, the resulting MR imaging features are not
constant, but rather evolve in a somewhat predictable fashion over time (87, 88). Although it is the age of a hematoma that most influences its MR imaging appearance, the application of such terms as acute, subacute, and chronic to the status of the hemorrhagic collection has not been consistent. In general usage, the term acute hematoma is used for a lesion hours to days old. A subacute hematoma is one that is 1 week to approximately 3 months old, and a chronic hematoma is one older than 3 months (89, 90).
constant, but rather evolve in a somewhat predictable fashion over time (87, 88). Although it is the age of a hematoma that most influences its MR imaging appearance, the application of such terms as acute, subacute, and chronic to the status of the hemorrhagic collection has not been consistent. In general usage, the term acute hematoma is used for a lesion hours to days old. A subacute hematoma is one that is 1 week to approximately 3 months old, and a chronic hematoma is one older than 3 months (89, 90).
KEY CONCEPTS
The MR imaging appearance of a hematoma is variable and is a function of the age of the lesion.
Extracranial hematomas are usually classified as acute, subacute, or chronic.
Acute hematoma (1 week or less) shows a signal intensity relatively similar to that of skeletal muscle on T1-weighted images and decreased signal intensity on T2-weighted images.
Subacute hematomas (approximately 1 week to 3 months old) demonstrate variably increased signal intensity on both T1- and T2-weighted spin-echo MR images.
Chronic hematomas reveal increased signal intensity on all spin-echo MR pulse sequences (similar to that seen in subacute lesions), with a hemosiderin-laden rind that may be quite extensive.
Chronic hematoma eventually shows low signal intensity on all pulse sequences.
Chronic hematomas may continue to expand over time due to osmotic pressure from blood breakdown products.
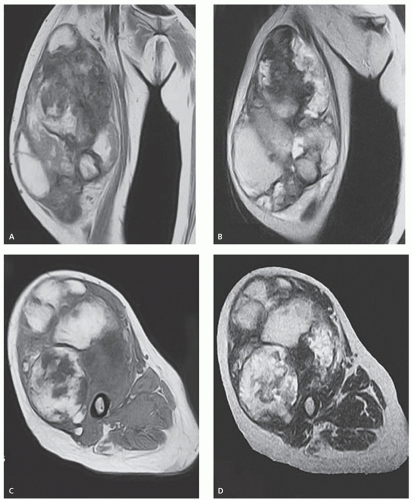
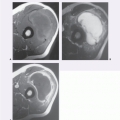
The MR imaging of extracranial hematomas was welldescribed by Rubin et al. (90) in a report of 20 cases. On spin-echo MR imaging, an acute hematoma shows signal intensity relatively similar to that of skeletal muscle on T1-weighted images, varying from slightly hyperintense to hypointense. A decreased signal intensity, less than that of skeletal muscle, is seen on T2-weighted images (Fig. 13.12). Acute hematomas are typically homogeneous, although considerable variability and a heterogeneous appearance may be seen; in addition, adjacent edema may be a prominent feature (Fig. 13.13).
Subacute hematomas (approximately 1 week to 3 months old) demonstrate homogeneous increased signal on both T1- and T2-weighted spin-echo MR images (Fig. 13.14). Early subacute lesions show an intermediateintensity center with a high signal intensity periphery (Fig. 13.15). A rind of decreased signal intensity caused by the accumulation of hemosiderin-laden macrophages may be seen and was noted in 9 (56%) of 16 subacute hematomas reviewed by Rubin et al. (90).
Chronic hematomas reveal increased signal intensity on all spin-echo MR pulse sequences, similar to that seen in subacute lesions. The rind of hemosiderin-laden tissue may be quite extensive, and eventually the lesion shows
signal intensity less than that of skeletal muscle on all pulse sequences (see Fig. 13.15). The time sequence for this transition is not established (see Fig. 13.14). In certain cases, a chronic hematoma may continue to enlarge. This phenomenon, termed chronic expanding hematoma, is likely caused by increased osmotic pressure due to the blood breakdown products and their effect on the permeability of the adjacent vessels (91, 92).
signal intensity less than that of skeletal muscle on all pulse sequences (see Fig. 13.15). The time sequence for this transition is not established (see Fig. 13.14). In certain cases, a chronic hematoma may continue to enlarge. This phenomenon, termed chronic expanding hematoma, is likely caused by increased osmotic pressure due to the blood breakdown products and their effect on the permeability of the adjacent vessels (91, 92).
The CT appearance of hematoma is also variable. Early lesions show increased attenuation compared with that of skeletal muscle (Figs. 13.13 and 13.15), whereas subacute to chronic lesions demonstrate an attenuation equal to or less than that of skeletal muscle (see Fig. 13.14) (89).
Care must be taken during imaging to differentiate a malignant soft tissue tumor with hemorrhage
from a hematoma. The presence of a tumor nodule or rim of tumor may be helpful in distinguishing a simple hematoma from a hemorrhagic neoplasm (87, 93). Gadolinium-enhanced imaging is also extremely useful in these circumstances to identify an underlying tumor nodule. This differentiation is often difficult because hematomas may organize, resulting in a complex appearance containing enhancing fibrovascular tissue (Fig. 13.16). Nevertheless, careful clinical correlation is needed, as is a healthy dose of skepticism. A history of “spontaneous hematoma” should always initiate a vigilant search for a hemorrhage associated with a neoplasm. If imaging is inconclusive, close follow-up is essential. Lenchik et al. (94) studied the CT scans of 44 patients with abnormalities of
the iliopsoas compartment (15 neoplasms, 21 abscesses, and 8 hematomas), to identify findings that would be useful to differentiate these processes. They noted that these processes could not be reliably distinguished on the basis of CT imaging findings. The most reliable CT feature for a hematoma was involvement of the entire muscle. The most reliable feature of neoplasm was irregular margins, but this was also common in abscesses and hematomas. Clinical enlargement of a soft tissue mass may be the result of necrosis and hemorrhage within a tumor (95). This enlargement is more common in large, high-grade sarcomas and may occur spontaneously, or as a result of chemotherapy (95). The differentiation between tumor growth and hemorrhage is especially important in patients undergoing chemotherapy, in order to establish clinical response.
from a hematoma. The presence of a tumor nodule or rim of tumor may be helpful in distinguishing a simple hematoma from a hemorrhagic neoplasm (87, 93). Gadolinium-enhanced imaging is also extremely useful in these circumstances to identify an underlying tumor nodule. This differentiation is often difficult because hematomas may organize, resulting in a complex appearance containing enhancing fibrovascular tissue (Fig. 13.16). Nevertheless, careful clinical correlation is needed, as is a healthy dose of skepticism. A history of “spontaneous hematoma” should always initiate a vigilant search for a hemorrhage associated with a neoplasm. If imaging is inconclusive, close follow-up is essential. Lenchik et al. (94) studied the CT scans of 44 patients with abnormalities of
the iliopsoas compartment (15 neoplasms, 21 abscesses, and 8 hematomas), to identify findings that would be useful to differentiate these processes. They noted that these processes could not be reliably distinguished on the basis of CT imaging findings. The most reliable CT feature for a hematoma was involvement of the entire muscle. The most reliable feature of neoplasm was irregular margins, but this was also common in abscesses and hematomas. Clinical enlargement of a soft tissue mass may be the result of necrosis and hemorrhage within a tumor (95). This enlargement is more common in large, high-grade sarcomas and may occur spontaneously, or as a result of chemotherapy (95). The differentiation between tumor growth and hemorrhage is especially important in patients undergoing chemotherapy, in order to establish clinical response.
The term Morel-Lavallée lesion is applied to a special form of chronic hematoma. The lesion, typically seen in the proximal thigh and trochanteric region, is thought to be the result of traumatic separation of the subcutaneous, fibrofatty tissue from the adjacent fascia, causing disruption of the rich vascular plexus involving the fascia in this area (96, 97, 98). These lesions are discussed in Chapter 12: Superficial Soft Tissue Masses.
Lymphoma
Lymphoma can involve the musculoskeletal system as part of disseminated systemic disease or as an isolated phenomenon. Soft tissue lymphoma is most commonly seen in association with disseminated disease; true primary lymphoma of soft tissue of any histologic subtype is extremely rare (99, 100). It is difficult to get accurate statistics, but primary lymphoma arising in muscle likely accounts for 1% or less of all lymphomas (101, 102). In a study of 75 cases of primary muscle lymphoma seen in consultation over 20 years at the Armed Forces Institute of Pathology, Lanham and colleagues (103) found patients to be older (usually older than 60 years), white (96% with no cases in blacks), with large cell non-Hodgkin
lymphoma. Lesions were most common in the thigh (33%), followed by the thoracoabdominal wall (29%), arm (15%), and lower leg (11%).
lymphoma. Lesions were most common in the thigh (33%), followed by the thoracoabdominal wall (29%), arm (15%), and lower leg (11%).
Patients with intramuscular lymphoma most often present with a palpable mass, simulating a soft tissue sarcoma. In patients with systemic disease, whole-body imaging evaluation with positron emission tomography (PET) or other modality will identify the full extent of the disease. MR imaging of the presenting mass in these cases often, although not invariably, shows abnormalities in the adjacent osseous structures or lymph nodes, suggesting the diagnosis or at least allowing system lymphoma to be included in the differential diagnosis (104, 105, 106). Clearly, primary soft tissue lymphoma may rarely present as an isolated soft tissue mass (99, 105, 107).
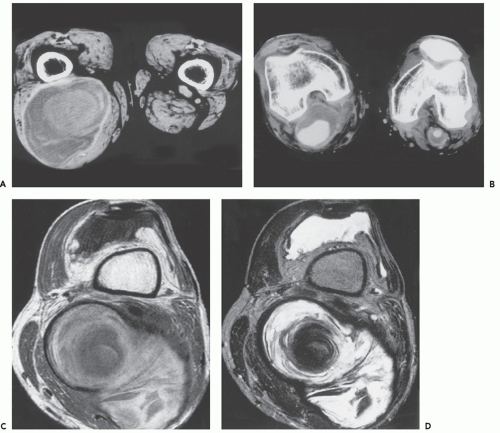
The MR imaging features of skeletal muscle lymphoma are similar in those patients with primary or secondary soft tissue disease. As a generalization, the diagnosis of muscle lymphoma should be suggested when there is tumor with relative preservation of the surrounding structures (108), diffuse muscle enlargement, and long segment or multicompartmental involvement (109, 110, 111, 112). Lesions generally demonstrate a signal intensity similar to or slightly greater than that skeletal muscle on T1-weighted images and intermediate on T2-weighted images, with diffuse enhancement, usually homogeneous or moderately heterogeneous, following contrast administration (Fig. 13.17)
(108, 109, 110). Thickening and enhancement of the adjacent fascia is also seen in the vast majority of patients, as is stranding of the adjacent subcutaneous tissue (109, 110).
(108, 109, 110). Thickening and enhancement of the adjacent fascia is also seen in the vast majority of patients, as is stranding of the adjacent subcutaneous tissue (109, 110).
KEY CONCEPTS
Intramuscular lymphoma is quite rare.
MR imaging features of skeletal muscle lymphoma are similar in patients with primary or secondary soft tissue disease. Findings include:
Diffuse muscle enlargement with long segment involvement.
Multiple muscle or multicompartmental involvement.
Signal intensity similar to or slightly greater than that of skeletal muscle on T1-weighted images.
Intermediate signal intensity on T2-weighted images.
Diffuse, usually homogeneous or moderately heterogeneous, enhancement.
At CT imaging, lesions will be homogeneous with an attenuation similar to or slightly less than that of skeletal muscle.
Ultrasound will show a relatively homogeneous, hypoechoic, hypervascular mass.
At CT imaging, lesions will be homogeneous, noncalcified, with an attenuation similar to or slightly less than that of skeletal muscle (Fig. 13.18) (110, 111, 113). At ultrasound, extranodal lymphoma will appear as a relatively homogeneous, hypoechoic, hypervascular mass (Fig. 13.18). Swelling of the muscle bundles and coarsening of the fibroadipose septa have also been reported (114, 115).
Melorheostosis (Soft Tissue)
Melorheostosis is a relatively rare bone dysplasia that was originally described by Leri and Joanny in 1922 (116). Osseous manifestations are frequently characteristic, with radiographs showing undulated cortical thickening having a classic “flowing candle wax” appearance (116, 117, 118, 119, 120, 121, 122). Patients are often symptomatic, with pain, contractures,
and limited range of motion. The lower limb is most frequently affected in a sclerotomal pattern (osseous distribution of a spinal sensory nerve), usually involving multiple contiguous osseous sites.
and limited range of motion. The lower limb is most frequently affected in a sclerotomal pattern (osseous distribution of a spinal sensory nerve), usually involving multiple contiguous osseous sites.
KEY CONCEPTS
Melorheostosis is a rare bone dysplasia with characteristic radiographic features.
Soft tissue masses are also a recognized feature, occurring in about 27% of cases.
Mineralized soft tissue masses are frequent and easily recognized on radiographs or CT.
Nonmineralized soft tissue masses have a CT attenuation similar to that of muscle.
On MR imaging, nonmineralized components may show focal areas with adipose tissue characteristics or reveal intermediate signal intensity on T1-weighted MR images and high signal on T2 weighting.
Contrast MR images typically show marked enhancement of the nonmineralized soft tissue components.
Soft tissue masses are a recognized feature of melorheostosis. Murray and McCredie reported soft tissue masses in 27% of their 30 patients with melorheostosis (117). The soft tissue masses are often, but not invariably, associated with areas of characteristic cortical thickening. There may also be associated fibrosis and resulting joint contracture. Desmoid tumors, as well as lymphatic and vascular anomalies and neoplasms, are also described in patients with melorheostosis (118, 123).
Pathologically, the soft tissue masses have a varied composition, although osseous and chondroid elements are the most prominent components (121). In addition,
fibrolipomatous and vascular tissue are also frequently present (118). Treatment of these soft tissue masses is based on the clinical assessment. Symptomatic lesions, particularly those causing joint contractures, are usually excised. Recurrence is infrequent.
fibrolipomatous and vascular tissue are also frequently present (118). Treatment of these soft tissue masses is based on the clinical assessment. Symptomatic lesions, particularly those causing joint contractures, are usually excised. Recurrence is infrequent.
The appearance of the soft tissue masses associated with melorheostosis on imaging is variable. Mineralized soft tissue masses are easily recognized on radiographs or CT scanning, and show markedly increased uptake of radionuclide on bone scintigraphy (116, 117, 118, 119, 120, 121, 122). Nonmineralized soft tissue masses associated with melorheostosis are more difficult to assess, and, if not recognized as a manifestation of the disease, may be confused with a more ominous process (124). Judkiewicz et al. (124) reviewed the imaging findings in 17 patients with melorheostosis. Soft tissue masses were noted in 9 patients (53%), 5 of which had CT scans that showed the lesions to be prominently mineralized in 80%.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree