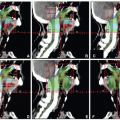
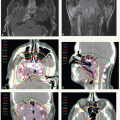
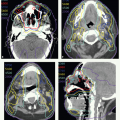
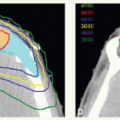
Modes of Therapy
PRIMARY RADIOTHERAPY
Key Points
External beam radiotherapy, generally using 6-MV x-rays with or without some components of electrons, has an important role in frontline therapy for patients with carcinoma of the oropharynx, larynx, or hypopharynx; its aim is to eradicate tumors while preserving organ structure and function.
Intensity-modulated radiation therapy (IMRT) has been widely adopted because of its ability to better target tumors and reduce late morbidity by diminishing the radiation dose to salivary glands and other critical organs.
Brachytherapy, alone or as a supplement to external beam therapy, can yield high tumor control rates with less toxicity in selected patients, but its use is decreasing with the increasing use of IMRT.
Chemotherapy is often given, either concurrently with radiation or before radiation, to improve control of locally advanced carcinomas.
Combination therapy with radiation and the anti-epidermal growth factor receptor antibody cetuximab has emerged as an alternative to chemoradiation, with a better toxicity profile, for the frontline treatment of patients with carcinoma of the oropharynx, larynx, or hypopharynx.
Surgery remains the preferred therapy for cancers of the oral cavity, paranasal sinuses, salivary glands, and thyroid and also for less common head and neck neoplasms.
Postoperative radiation, alone or combined with concurrent chemotherapy when indicated, is an established adjuvant treatment for patients with adverse surgical pathologic features, such as multiple positive lymph nodes, extranodal disease extension, positive surgical margins, and perineural invasion, among others.
External Beam Irradiation (Teletherapy)
Primary frontline radiotherapy is generally preferred for the treatment of patients with carcinoma of the oropharynx, larynx, or hypopharynx. Such therapy is most often delivered through external beam irradiation with 6- or 18-MV x-rays, 6- to 20-MeV electron beams, or a combination of both photons and electrons of proper beam energies. The choice of the beam type and energy is based on the location and geometric parameters of the target volumes. Occasionally, orthovoltage x-rays can be useful, for example, for treatment of skin cancers or for intraoral cone therapy for accessible, well-circumscribed tumors of the oral cavity or anterior oropharynx.
The initial target volume for external beam therapy includes both the gross tumor, as determined by clinical examinations and diagnostic imaging, and the potential routes of subclinical (microscopic) disease spread. Until about a decade ago, a shrinking field technique was widely used in which the target volume is reduced after a dose sufficient to sterilize subclinical disease is reached to deliver an additional boost dose to the demonstrable gross tumor. The boost dose may be given after or concomitantly with the initial large-field target volume irradiations. In the latter
scenario, the boost irradiations are administered as second daily fractions.1
scenario, the boost irradiations are administered as second daily fractions.1
The common practice in the United States until the late 1990s was to administer primary radiotherapy in 2-Gy fractions, once a day, 5 days a week, to total doses ranging from 66 to 70 Gy depending on the tumor stage and site. As summarized in Chapter 1, intensive clinical investigations conducted during the past 3 decades revealed the superiority of hyperfractionation (e.g., 81.6 Gy given in 1.2 Gy per fractions, twice a day with 6-hour intervals, over 7 weeks) and two types of accelerated fractionation—concomitant boost (54 Gy given in 30 fractions over 6 weeks, plus an 18-Gy boost dose given in 1.5-Gy fractions as second daily fractions during the last 2.5 weeks) and six fractions of 2 Gy per week regimens—in yielding local-regional control.2,3 Consequently, many centers have adopted one of these regimens as the current standard for the treatment of intermediate-stage head and neck carcinomas or locally advanced cancers in patients who are not medically fit to receive chemotherapy.
Intensity-Modulated Radiation Therapy
As presented in Chapter 1, advances in computing and engineering technologies have greatly improved the flexibility and precision of aiming radiation beams at irregular volumes. Such precision radiotherapy can be achieved with intensity-modulated photon beams in IMRT. Intensity-modulated proton therapy is still in the developmental stage.
With IMRT, all target volumes are irradiated during every radiation session but, with a properly shaped dose gradient, a higher dose per fraction (e.g., 2 Gy) is delivered to the clinical target volume (CTV), which encompasses the gross tumor (primary lesion and involved nodes) with 1- to 1.5- cm margins (CTVHD), a lower elective dose (e.g., 1.6 Gy per fraction) to subclinical disease target volumes (CTVED), and, when desired, an intermediate dose (e.g., 1.8 Gy per fraction) to intermediary volumes (CTVID) surrounding the CTVHD. It should be noted, however, that in addition to delivering radiation in smaller fractions, the overall radiotherapy time for treating subclinical disease is prolonged from the conventional 5 weeks (50 Gy in 25 fractions) up to 6 to 7 weeks (30 to 35 fractions). Therefore, it is prudent to adjust the dose to the electively irradiated regions to correct for these changes. Consequently, when IMRT is given in 6 weeks to deliver a total dose of 66 Gy in 30 fractions to CTVHD such as in the treatment of T1 to T2 oropharyngeal carcinoma, we currently prescribe 54 Gy (1.8 Gy per fraction) to the elective volume. When a total dose of 70 Gy is given in 35 fractions (2.0 Gy per fraction) to CTVHD for treatment of larger tumors, we prescribe 56 Gy (1.6 Gy per fraction) to CTVED and 63 Gy to CTVID. In the latter scenario, IMRT can be given in conventional fractionation over 7 weeks by delivering five daily fractions per week or in accelerated fractionation over 6 weeks by administering six fractions per week (usually by prescribing two fractions a day, with a 6-hour interfraction interval, 1 day a week, for 5 weeks), a schedule modeled after the regimen used by Danish investigators.4 An alternative strategy to complete treatment in 6 weeks is by using a concomitant boost-type regimen1 in which all CTVs receive daily fractions of 1.8 Gy, five fractions per week for 6 weeks and the CTVHD receives second daily fractions of 1.8 Gy each during the last 10 treatment days. The concomitant boost-type regimen requires two dosimetric plans but has the advantage of having generally smaller volumes receiving radiation doses beyond the prescribed level (“hot spots”).
Brachytherapy
Brachytherapy is usually combined with external beam therapy. The rationale for this combination is that areas at risk of harboring subclinical disease are irradiated with external beams to a dose sufficient to sterilize microscopic deposits and that the gross lesion is boosted with a brachytherapy system to higher doses. This approach results in exposing a smaller volume of normal tissues to high radiation doses. Therefore, if applied appropriately by an experienced team, high tumor control rates can be obtained with generally low treatment morbidity.
Brachytherapy is used in the form of interstitial implants (e.g., for primary cancer of the base of tongue or tonsillar fossa extending into the base of tongue), molds (e.g., for lesions of the hard palate), or intracavitary applicators (e.g., for recurrent nasopharyngeal cancer).
In selected situations, brachytherapy is used alone. Examples of such applications include treatment of superficial lip cancers, small nasal vestibule neoplasms, and alveolar ridge carcinomas.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree