Despite the recognized limitations of 18 Fluorodeoxyglucose positron emission tomography (FDG-PET) in brain tumor imaging due to the high background of normal gray matter, this imaging modality provides critical information for the management of patients with cerebral neoplasms with regard to the following aspects: (1) providing a global picture of the tumor and thus guiding the appropriate site for stereotactic biopsy, and thereby enhancing its accuracy and reducing the number of biopsy samples; and (2) prediction of biologic behavior and aggressiveness of the tumor, thereby aiding in prognosis. Another area, which has been investigated extensively, includes differentiating recurrent tumor from treatment-related changes (eg, radiation necrosis and postsurgical changes). Furthermore, FDG-PET has demonstrated its usefulness in differentiating lymphoma from toxoplasmosis in patients with acquired immune deficiency syndrome with great accuracy, and is used as the investigation of choice in this setting. Image coregistration with magnetic resonance imaging and delayed FDG-PET imaging are 2 maneuvers that substantially improve the accuracy of interpretation, and hence should be routinely employed in clinical settings. In recent years an increasing number of brain tumor PET studies has used other tracers (like labeled methionine, tyrosine, thymidine, choline, fluoromisonidazole, EF5, and so forth), of which positron-labeled amino acid analogues, nucleotide analogues, and the hypoxia imaging tracers are of special interest. The major advantage of these radiotracers over FDG is the markedly lower background activity in normal brain tissue, which allows detection of small lesions and low-grade tumors. The promise of the amino acid PET tracers has been emphasized due to their higher sensitivity in imaging recurrent tumors (particularly the low-grade ones) and better accuracy for differentiating between recurrent tumors and treatment-related changes compared with FDG. The newer PET tracers have also shown great potential to image important aspects of tumor biology and thereby demonstrate ability to forecast prognosis. The value of hypoxia imaging tracers (such as fluoromisonidazole or more recently EF5) is substantial in radiotherapy planning and predicting treatment response. In addition, they may play an important role in the future in directing and monitoring targeted hypoxic therapy for tumors with hypoxia. Development of optimal image segmentation strategy with novel PET tracers and multimodality imaging is an approach that deserves mention in the era of intensity modulated radiotherapy, and which is likely to have important clinical and research applications in radiotherapy planning in patients with brain tumor.
Classification, epidemiology, management, and outcome of brain tumors: the salient points
Intracranial brain neoplasms can be classified as primary brain tumors and metastatic neoplasms from primary extracranial malignancies. The latter is far more common than the former; according to an American Cancer Society estimate, more than 100,000 people die every year with symptomatic intracranial metastases while death occurs due to primary brain tumors in approximately 12,760 of the 20,500 new cases diagnosed. Primary central nervous system (CNS) tumors account for about 1% to 2% of all malignancies. The basic classification of primary brain neoplasms by the World Health Organization (WHO) relies on their cellular origin. The incidence of primary brain neoplasms varies among subtypes, with the most common primary brain neoplasms in adults being gliomas, which account for about 45% to 50%, and meningiomas, comprising about 15% of all primary brain tumors, all other types being less common.
Gliomas are classified into astrocytomas, oligodendrogliomas, mixed oligoastrocytomas, ependymal tumors, and tumors of the choroid plexus based on histologic characteristics. Grade of malignancy is generally assigned according to the WHO criteria, which are primarily based on nuclear atypia, mitotic activity, endothelial/microvascular proliferation, and necrosis. The prognosis of primary brain tumors is primarily based on 3 factors: (1) histologic characteristics (subtype and grade of the tumor), (2) age, and (3) performance status. The other poor prognostic parameters for gliomas include presence of focal neurologic deficits, mental changes, seizure, symptoms of neoplastic expansion, and contrast enhancement on computed tomography (CT). However, the precise value of some of these factors is still unclear. Therefore, using an objective marker to determine patient’s prognosis is beneficial, as this helps guide therapeutic decision making for this unpredictable malignancy.
Low-grade gliomas histopathologically encompass pilocytic astrocytoma (a grade I tumor), astrocytoma (a grade II tumor), and oligodendroglioma (a grade II tumor). In general, the low-grade tumors affect younger patients (mean age: fourth decade) compared with the high-grade gliomas (mean age of presentation at the sixth decade of life). The most fatal and common primary brain neoplasm is the glioblastoma multiforme (GBM), which accounts for 45% to 50% of all gliomas and corresponds to WHO grade IV. Despite aggressive multimodal treatment regimens of conventional therapies (surgery, radiation, and chemotherapy), the disease invariably leads to death over months or years, with a median survival of 1 year. Combination of radiotherapy and chemotherapy with temozolomide has recently been shown to improve survival in patients with newly diagnosed glioblastoma, with a 2-year survival rate of 26.5% with radiotherapy plus temozolomide and 10.4% with radiotherapy alone. The remaining high-grade tumors include anaplastic tumors (astrocytoma and oligodendroglioma) and correspond to grade III, with a median survival of 2 to 3 years.
A complex series of molecular events occur during tumor growth resulting in deregulation of the cell cycle, alterations in apoptosis and cell differentiation, neovascularization, and tumor cell migration and invasion into the normal brain parenchyma. Genetic alterations also play an important role in the development of glioma including a loss, mutation, or hypermethylation of a tumor suppressor gene such as PTEN or p53, or other genes involved in the regulation of the cell cycle. During progression from low-grade to high-grade, stepwise accumulation of genetic alteration occurs. Growth of certain tumors seems to be related to the presence of viruses and familial diseases that accelerate the progression of molecular alterations, or to exposure to environmental chemicals, pesticides, herbicides, and fertilizers.
Positron emission tomography in brain tumors: what do the oncologists desire?
The exact delineation of the tumor volume, the characterization of the important tumor characteristics in its entirety, and assessment of the tumor response to the ongoing treatment regimen are necessary for successful management of these patients. In the current practice, initial imaging is usually done with contrast/enhanced CT or magnetic resonance (MR) imaging, which provides excellent information about lesion anatomy. However, follow-up of primary brain tumors after surgery, chemotherapy, and particularly radiation is often difficult, as these morphologic imaging modalities are usually not able to differentiate recurrent tumor and radiation necrosis. In all tumor types, diagnosing radiation injuries and differentiating them from tumor recurrence pose a potential diagnostic challenge because the accurate diagnosis has important implications for the patient’s management. Also, an optimal understanding of tumor biology is crucial for the development of specific molecular therapies that specifically target the neoplasm and reduce patient morbidity and mortality.
Positron emission tomography in brain tumors: what do the oncologists desire?
The exact delineation of the tumor volume, the characterization of the important tumor characteristics in its entirety, and assessment of the tumor response to the ongoing treatment regimen are necessary for successful management of these patients. In the current practice, initial imaging is usually done with contrast/enhanced CT or magnetic resonance (MR) imaging, which provides excellent information about lesion anatomy. However, follow-up of primary brain tumors after surgery, chemotherapy, and particularly radiation is often difficult, as these morphologic imaging modalities are usually not able to differentiate recurrent tumor and radiation necrosis. In all tumor types, diagnosing radiation injuries and differentiating them from tumor recurrence pose a potential diagnostic challenge because the accurate diagnosis has important implications for the patient’s management. Also, an optimal understanding of tumor biology is crucial for the development of specific molecular therapies that specifically target the neoplasm and reduce patient morbidity and mortality.
Positron emission tomography: how has it addressed these needs?
The interest in positron emission tomography (PET) in brain tumor stems from its potential ability to image a wide range of biochemical processes that are critical for understanding the pathophysiology of brain neoplasms, and thereby can play a significant role in developing and monitoring targeted therapies. Different radiotracers have been used with PET to help in the diagnosis and management of patients with brain neoplasms, and these are summarized in Table 1 .
| Principal Class of PET Tracer and Molecular Mechanism Involved | Name of Tracer | |
|---|---|---|
| A | Glucose metabolism | Fluorodeoxyglucose (FDG) |
| B | Amino acid analogues | [ 11 C]Methionine (MET), fluoroethyl- l- tyrosine (FET) and l- 3, 4-dihydroxy-6-[ 18 F]fluorophenylalanine (FDOPA), l- 1-[ 11 C]tyrosine (TYR), and l- 3-[ 18 F]fluoro-α-methyltyrosine (FMT) |
| C | Radiolabeled cell membrane components | [ 11 C]Choline PET (CHO) |
| D | Radiolabeled nucleosides | [ 18 F]Fluorothymidine (FLT) |
| E | Hypoxia Imaging Tracers | [ 18 F]Fluoromisonidazole, [ 18 F]EF5 |
| F | Somatostatin receptor imaging tracers | [ 68 Ga]DOTA-TOC PET |
Depending on the radiotracer, various molecular processes can be visualized and quantified by PET, which makes it an attractive modality for studying tumor biology of gliomas and other brain neoplasms. Radiolabeled 2-[ 18 F]fluoro-2-deoxy- d -glucose (FDG), methyl-[ 11 C]- l -methionine (MET), and 3-deoxy-3-[ 18 F]fluoro- l -thymidine (FLT) are taken up by proliferating gliomas depending on tumor grade as a reflection of increased activity of membrane transporters for glucose, amino acids, and nucleosides, respectively. More recently, methyl-[ 11 C]choline (CHO) has been introduced as a novel tracer to evaluate proliferating cell membrane, as is discussed later in this article. PET has been also used to assess the expression of endogenous or exogenous genes coding for enzymes or receptors by measuring the accumulation or binding of the respective enzyme substrates or receptor binding compounds.
Continuous developments in PET provide new insights into the diagnosis, classification, and pathophysiology of brain neoplasms. As such, PET has played an increasingly important role in the staging of brain neoplasms, image-guided therapy planning, and treatment monitoring. Multimodality imaging has brought about a new perspective into neuro-oncology, as PET complements the conventional anatomic imaging modalities of CT and MR imaging. In selected situations, PET is used in conjunction with CT and MR imaging to better define the extent of neoplasm.
Recent research has concentrated on the fusion of PET and MR imaging technologies into one single machine. The goal of this development is to integrate the PET detectors into the MR imaging scanner, which would allow simultaneous data acquisition, resulting in combined functional and morphologic images with excellent soft tissue contrast, spatial and temporal resolution, and improved coregistration of the fused images. Because advanced MR imaging techniques, such as perfusion weighted imaging, diffusion weighted imaging (DWI), blood oxygenation level dependent (BOLD) imaging, and proton magnetic resonance spectroscopy (MRS) provide physiologic and metabolic information, fusion of PET with MR imaging may provide more insight into the pathophysiology of brain neoplasms in vivo.
The following discussion addresses the most commonly used agents in PET imaging of brain tumors.
Fluorodeoxyglucose
Although there are certain advantages for newer radiotracers, the vast majority of clinical PET studies are still based on administering FDG, an analogue of glucose. Because of its relatively simple synthesis, long half-life (approximately 2 hours), and well-understood mechanism of uptake, FDG is the radiotracer commonly used in neuro-oncology.
Malignant cells generally have enhanced glucose metabolism compared with nonmalignant cells, and therefore exhibit increased glycolytic activity. The increase in glucose metabolic rate is not simply related to the accelerated tumor growth but also to malignant transformation and increased membrane glucose transport capability. There is a significant increase in the number of functional glucose transporters at the transformed cell’s surface, and nearly all mitogens and cellular oncogenes activate glucose transport. Six mammalian glucose transporters have been identified, and overexpression of both GLUT-1 and GLUT-3 has been demonstrated in brain tumors, with a higher ratio of GLUT-3 seen in more aggressive neoplastic lesions.
FDG is metabolized similarly to glucose, and as FDG enters a cell via glucose transporter proteins, it competes with glucose for hexokinase and is phosphorylated. In contrast to glucose-6-phosphate, however, FDG-6-phosphate is metabolically trapped within tumor cells in proportion to the glucose metabolic rate, and thus PET can detect its accumulation in these cells over time. The FDG uptake into malignant cells is a consequence of a tumor cell’s increased expression of glucose transport and glycolytic activity.
Clinical applications of FDG-PET in neuro-oncology
Tumor Detection with FDG-PET
Distinguishing nonneoplastic from neoplastic lesions can be challenging when based on anatomic imaging. Due to its poor spatial resolution, FDG-PET has been shown to be of limited value in distinguishing between neoplastic and nonneoplastic ring-enhancing intracranial lesions, as observed on MR imaging. High FDG uptake may occur in cases of neoplasm, brain abscess, and in acute inflammatory demyelination. Multiple reports have shown that lesions with a high concentration of inflammatory cells, such as neutrophils and activated macrophages, also show increased uptake of FDG that can be mistaken for malignant neoplasm. In general, inflammatory cells often exhibit lower levels of FDG uptake compared with malignant cells. However, despite low levels of FDG uptake by inflammatory cells in the resting state, glucose metabolic activity of these cells can increase dramatically in the activated state, making the distinction from tumor difficult. In addition to the neoplastic cells, inflammatory cells also have increased expression of glucose transporters, particularly after cellular stimulation by multiple cytokines. Cytokines and growth factors have been shown to acutely increase the affinity of glucose transporters in inflammatory cells to deoxyglucose, and this upregulation involves both tyrosine kinases and protein kinase C activity. It is postulated that the newly formed granulation tissue around a neoplasm contains high concentrations of macrophages and thus can show a higher uptake of FDG than the viable neoplastic cells.
Delineation of Tumor
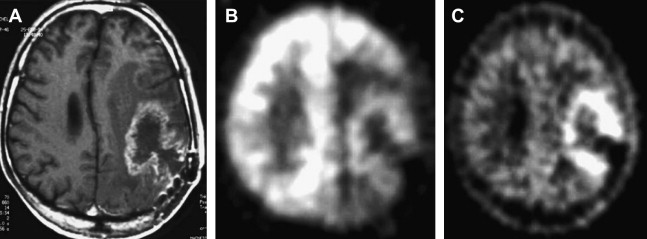
Both high-grade gliomas and gray matter structures take up FDG avidly. Thus, when tumors are in or near gray matter, it may be difficult to distinguish between the two. Two maneuvers seem to greatly enhance the performance of FDG-PET imaging in brain tumors: (1) coregistration with MR imaging and (2) delayed PET Imaging. An MR image delineates the area of interest precisely, and any increased uptake more than that of background in this region should be considered to reflect an active process. The importance of delayed imaging has recently been stressed when it was observed that FDG-PET imaging 3 to 8 hours after injection can improve the distinction between tumor and normal gray matter. The rational approach to interpreting brain FDG-PET is detailed later in this article. It has been demonstrated that the rate constant of FDG-6-phosphate degradation was not significantly different between tumor and normal brain tissue at early imaging times, but was lower in tumor than normal brain tissue at extended time intervals between FDG administration and PET data acquisition. This finding suggests that greater FDG-6-phosphate degradation at delayed time points may be responsible for higher elimination of FDG from normal tissue relative to neoplasm, which improves the contrast between the tumor and the background activity ( Fig. 1 ).
In a group of 10 patients with astrocytomas (WHO grade II and III), Herholz and colleagues found that cell density, but not nuclear polymorphism, correlated significantly with FDG uptake. Standardized uptake values (SUV) in the brain do not correlate well with regional metabolic rates of glucose use (MRGlu), and are less effective in characterizing primary brain neoplasms than neoplasm-to-white matter or neoplasm-to-cortex ratios.
As noted earlier, FDG uptake is not specific for cancer, as FDG accumulation has been observed in inflammatory cells and granulation issue. However, it must be noted that FDG uptake is decreased in the gray matter adjacent to the brain tumor on PET images due to surrounding edema ( Figs. 2 and 3 ).
Tumor Grading with FDG-PET
Although some studies have failed to show a relationship between FDG uptake and Ki-67 index, a histologic index for cell proliferation, in general, low-grade gliomas and high-grade gliomas demonstrate different degrees of FDG uptake. A glioma-to-white matter ratio of greater than 1.5 and a glioma-to-gray matter ratio of greater than 0.6 have been found to indicate high-grade gliomas with high sensitivity (94%) and limited specificity (77%). In general, the low-grade and high-grade glioma have FDG metabolic activity similar to white matter and gray matter, respectively. Kaschten and colleagues proposed a cutoff value of 0.8 and 1.1 for tumor-to-mean cortical uptake to differentiate grade II from grade III and grade III from grade IV gliomas, respectively. Several other reports have also shown significant correlation between glioma grade and FDG uptake, whereas others have failed to show such an association. The underlying mechanisms leading to glucose hypermetabolism in high-grade gliomas are likely related to increased energetic demands related to proliferative processes, increased expression of glucose transporters in response to oncogene expression, or the deregulation of the hexokinase enzymatic activity. Oligodendroglial tumors harboring combined 1p and 19q chromosomal deletions are characterized by a favorable prognosis and response to treatment. In a recent study, investigators reported the potential of FDG uptake to predict 1p/19q loss preoperatively. Positive FDG uptake was identified in 6 of 8 grade II gliomas with 1p/19q loss, but in none of the 8 grade II gliomas without 1p/19q loss. This finding needs to be investigated in a larger sample of patients.
Disease Prognosis with FDG-PET
FDG-PET imaging of brain neoplasms provides important information on neoplastic grade and patient prognosis. High FDG uptake in a previously known low-grade tumor is a reliable indicator of anaplastic transformation. In high-grade gliomas, some studies have claimed that FDG-PET has predictive value of survival independent from the histologic classification. Di Chiro examined 45 patients with proven high-grade brain tumors after surgery, radiation, and chemotherapy. Poorly differentiated tumors showed significantly higher glucose metabolism than more differentiated ones. The calculation of the ratio of metabolic values (tumor compared with the contralateral normal brain parenchyma) revealed that a ratio of greater than 1.4 was associated with a poor prognosis (median survival 5 months), whereas patients with a ratio of less than 1.4 had a median survival of 19 months. Alavi and colleagues came to similar conclusions using 18 FDG-PET in 29 patients with primary brain tumors. Patients with hypermetabolic tumors had a median survival of 7 months after PET scan, compared with 33 months for those with hypometabolic lesions on FDG-PET ( P = .0007). Of the 23 patients with high-grade tumors, 9 patients with hypometabolic lesions had a 1-year survival of 78%, whereas the group with hypermetabolic lesions had a 1-year survival of only 29%. These investigators concluded that glucose metabolic studies may provide an independent measure of the aggressiveness of a brain tumor, and may supplement pathologic grading. In another study of a pediatric population, 38 children with primary brain tumors were evaluated. Four grade IV tumors had a mean index of 4.27 ± 0.5, 4 grade III tumors had a mean index of 2.47 ± 1.07, 10 grade II tumors had a mean index of 1.34 ± 0.73, and 8 of 12 grade I tumors had a mean index of −0.31 ± 0.59. Eight patients with no histologic confirmation had a mean index of 1.04. For these 34 tumors, FDG uptake was positively correlated with the grade of malignancy ( n = 34; r = 0.72; P <.01), and for the 26 histologically classified tumors ( n = 26; r = 0.89; P <.01).
Barker and colleagues studied the prognostic value of FDG-PET in 55 patients with malignant glioma. In univariate analysis, the FDG-PET score was a significant predictor of survival time after FDG-PET scanning ( P = .005). Median survival was 10 months for patients with FDG-PET uptake scores of 2 or 3 and 20 months for those with low uptake scores of 0 or 1. Padma and colleagues also observed similar findings, and the median survival of patients with high uptake scores in their study was 11 months as compared with 28 months in patients with low uptake scores. Survival differed significantly between patients with low versus high uptake of FDG ( P = .001).
Other groups have reported significant correlation between FDG-PET score and survival in patients with malignant gliomas imaged at various times in the course of their disease. Patronas and colleagues reported median survival of 5 and 19 months in patients with hypermetabolic and hypometabolic lesions, respectively ( P <.001). Not all studies have confirmed the high predictive ability of FDG-PET with regard to grade of pathology or survival. Tyler and colleagues found variable FDG uptake rates without correlation to neoplastic grade and size, and low FDG uptake in several patients with high-grade gliomas. Janus and colleagues found that decreased uptake of FDG suggested prolonged survival in their study of 30 patients with primary brain neoplasms, but that increased uptake of FDG did not predict survival. Rozenthal and colleagues found that neither the baseline glucose uptake ratio nor the visual tumor grade accurately predicted length of survival. This lower sensitivity and specificity of FDG-PET in the prediction of pathologic grade and survival may be due volume averaging from necrotic portions of a heterogeneous neoplasm and surrounding edema. Moreover, cystic and calcified regions are also hypovascular.
Differential Diagnosis of Enhancing Brain Neoplasms with FDG-PET and Other PET Tracers
The role of FDG-PET is limited in the differential diagnosis of solitary intracranial ring-enhancing lesions. FDG is nonspecific in that its uptake is increased in inflammatory/infectious lesions as well as in certain benign lesions like pituitary adenomas and choroid plexus papillomas. Also, metastases from various non-CNS primary tumors appear to have more variable FDG uptake. A negative FDG-PET could be helpful to exclude a glioblastoma. The diagnostic value of O -2-[ 18 F]fluoroethyl- l- tyrosine (FET)-PET and MET-PET does not seem to be superior to that of FDG-PET, both of which have been reported to show uptake in the area of macrophage infiltration at the rim of brain abscesses. A single study has demonstrated a significant difference in fluorocholine uptake between benign lesions, high-grade glioma, and the metastatic lesions. The other relatively benign neoplasms with a high FDG uptake include pilocytic astrocytoma and ganglioglioma. Pilocytic astrocytomas have a good prognosis despite exhibiting high FDG uptake due to the presence of metabolically active fenestrated endothelial cells.
It is also possible to differentiate grade I from grade II to III meningiomas using FDG-PET. Meningiomas are usually benign, curable neoplasms but can occasionally recur and demonstrate aggressive behavior. Di Chiro found that the glucose metabolism of meningiomas correlates with tumor growth and aggressive behavior. In this study an atypical meningioma had the highest metabolic rate greater than that of cerebral cortex. Hemangiopericytomas are richly vascularized mesenchymal tumors that are derived from pericytes. Kracht and colleagues observed low glucose use in a case of hemangiopericytoma despite high cellularity and proliferation rate.
Efficacy of Therapy: Differentiating Post-Therapy Radiation Necrosis from Residual/Recurrent Brain Tumor
Differentiation of post-therapy radiation necrosis from residual/recurrent brain neoplasm remains a challenging task. This differential diagnosis seems particularly critical in the early and late phases after the completion of radiation therapy. The effect of radiation appears more pronounced when it is combined with chemotherapy for treating high-grade gliomas. A wide range of FDG-PET performance has been reported in different studies in differentiating radiation necrosis from recurrent tumor, and this can be primarily attributed to the following: (a) timing of PET study following radiation ( Table 2 ), (b) type of radiation administered; and (c) type of tumor. All these factors can lead to considerable overlap of FDG uptake between these 2 entities. Residual/recurrent neoplasm can have similarly varied degrees of metabolic activity that also can frequently be lower than that of normal brain. The accuracy appears better in gliomas compared with metastatic lesions and is worse in lesions treated with stereotactic radiosurgery. Whereas there is no clear-cut guideline with regard to optimal time for performing FDG-PET after radiation, the general recommendation is that FDG-PET should not be performed before 6 weeks after the completion of radiation treatment for this purpose.
| Types of Radiation Injuries | |||
|---|---|---|---|
| Type of Injury | Time Interval after Irradiation | Pathology | Prognosis |
| Acute | Hours to weeks |
| Good (reversible) |
| Early delayed | Weeks to months | Demyelination | Good (spontaneously reversible) |
| Late | Months to years | Liquefactive or coagulative necrosis |
|
Varying degrees of background metabolic activity can be observed in the area of treated brain as well as the recurring tumors, and hence in the lesion to contralateral normal brain tissue uptake ratio or the absolute uptake value. This factor has led to decidedly mixed results in various studies. Whereas in some studies FDG-PET has been found not to be ideal to evaluate residual/recurrent neoplasm after therapy, others have reported promising results using receiver operating characteristics analysis. In an early report, Patronas and colleagues demonstrated that metabolic imaging with FDG can differentiate recurrent brain tumor from radiation necrosis. The same group, in another study with brain tumors, demonstrated the feasibility of brain tumor imaging with FDG-PET and showed that it is more accurate than contrast CT for tumor grading. The effects of radiation and chemotherapy can be visualized by FDG-PET only after several weeks, with a possible transient increase of FDG uptake in the initial phase, most likely due to infiltration of macrophages. At further follow-up, however, recurrent neoplasm and progression from low-grade to high-grade glioma can be visualized by the appearance of new hypermetabolic sites. Glantz and colleagues demonstrated that FDG-PET imaging in brain tumor patients was not only superior to contrast CT in identifying early recurrence but also a better predictor of outcome than surgical biopsy results. In this study the vast majority of patients (19 of 20) with hypermetabolic lesions in areas of prior resection had early tumor recurrence. All 12 patients with hypometabolic abnormalities revealed radiation necrosis.
Overall performance that has been reported indicates that FDG-PET had a sensitivity of 81% to 86% and a specificity of 40% to 94% for distinguishing between radiation necrosis and residual/recurrent neoplasm. It should be emphasized that in patients receiving corticosteroids, evaluation with FDG-PET might be hampered by a generalized reduction in cerebral metabolic rate for glucose. Coregistration of FDG-PET images with MR imaging and delayed imaging greatly improves the accuracy of FDG-PET in this setting ( Fig. 4 ), and this is addressed in detail later in this article.
FDG-PET Imaging for Planning Stereotactic Biopsy
In the histologically heterogeneous gliomas, stereotactic biopsy aims for tumor sites with the highest tumor grade. FDG-PET guided stereotactic brain biopsy has proven to be useful for improved delineation of anaplastic regions and better identification of neoplastic residues, thereby increasing the diagnostic yield of brain biopsy. It is well known that low-grade and high-grade regions may be present within the same tumor, with components that include varying degrees of cellular and nuclear pleomorphism, mitotic activity, vascular proliferation, and necrosis. These regional variations cannot be reliably distinguished on conventional anatomic imaging such as MR imaging or CT, even with intravenous contrast administration. Accurate grading and diagnosis are especially important for directing the therapeutic approach and providing the prognosis in patients with nonresectable neoplasms. Several novel approaches have been investigated, including: (a) combined structure-function approach using PET and MR imaging; (b) combining FDG and an amino acid tracer (like [ 11 C]methionine); and (c) using coregistered MR imaging and amino acid tracers like FET. All these multimodality approaches are useful in accurately defining the disease extent and viability. Integration of MR imaging with PET provides complementary information that helps in the assessment of neoplastic extent and surgical planning better than either technique alone. This combined information has been exploited in accomplishing maximum neoplastic resection. Also, at follow-up, recurrent neoplasm and progression from low-grade to high-grade glioma can be visualized by the new appearance of hypermetabolism, and this can aid in guiding the site of biopsy.
FDG-PET in Central Nervous System Lymphoma
Primary CNS lymphomas, like the other extracerebral types, are highly proliferative and untreated tumors show avid FDG uptake. However, prior chemotherapy as well as dexamethasone treatment can cause substantial reduction in the FDG uptake and should be borne in mind while interpreting scan in this setting. In human immunodeficiency virus–infected patients, FDG-PET can help to discriminate CNS lymphoma from toxoplasmosis with a high degree of accuracy (80%–95%).
Pitfalls of FDG-PET in Brain Tumors and Improving the Accuracy of Interpretation: The Rational Approach
Compared with other organ systems, the brain presents unique challenges because of the high background glucose metabolism of normal gray matter structures. As noted earlier, the diagnostic limitations of FDG-PET related to the high physiologic glucose metabolism of normal brain tissue can lead to low sensitivity for detecting low-grade tumors and recurrent high-grade tumors in posttreatment settings. Uptake can at times be similar or even less than the normal gray matter. Hence, attempts have been made to improve the sensitivity of FDG-PET for lesion detection. Two steps seem critical for improving the accuracy of interpretation: (a) MR imaging coregistration and (b) delayed imaging whereby the tumor to normal brain uptake ratio increases with time following injection. Coregistration of FDG-PET images with MR imaging greatly improves the performance of FDG-PET. If the PET/MR imaging coregistration software is not available, it is important to review MR imaging along with the FDG-PET images at the same time. Because residual/recurrent neoplasm may show FDG uptake equal to or lower than that of the normal cortex, reference to the MR imaging delineates the area of interest and aids in improved accuracy. A reasonable approach for interpretation that can be useful is that in the area of interest on MR imaging, any FDG uptake higher than the expected background level in the adjacent brain may be considered recurrent neoplasm, even though the uptake may be equal to or less than that in normal cortex. In addition, with delayed imaging there is enhanced tumor to normal brain uptake ratio compared with the baseline image, which can further substantiate the presence of disease. A recent report has also advocated the use of coregistered FDG-PET and MR imaging to determine the residual/recurrent neoplasm viability by enhancing regions of the tumor. Another major limitation of PET is its relatively low specificity.
Promising “Non-FDG” PET Tracers for Brain Tumor Imaging and Their Current Status with Regard to Clinical Applications
In the past few years there have been an increasing number of brain tumor PET studies to circumvent the shortcomings of FDG-PET imaging, which apply other positron labeled radiotracers. These studies are enumerated in Table 1 .
The most significant advantage of these tracers is related to the markedly lower background activity in normal brain tissue compared with FDG, and relatively avidity toward the brain neoplasms that enables the detection of smaller and low-grade tumors (primary or recurrent). Among these tracers the labeled methionine, tyrosine, and thymidine tracers are relatively more widely investigated, as are the hypoxia imaging tracers, owing to their ability to define tumor biology and thereby guide the course of therapy.
Promising Role of Amino Acid PET Tracers
Proteins play a critical role in nearly every biologic process within the body. Amino acids, the building blocks of proteins, are either produced via metabolic processes or obtained via dietary ingestion. Fundamental to their ability to be a target of metabolic imaging, they can serve as components in metabolic cycles, many of which are upregulated in cells with increased proliferative activity, such as in the setting of cancer. Amino acids enter cells either through passive diffusion or, more commonly, active diffusion, particularly via the l- amino acid transporter. What makes amino acids attractive for the most common form of radiolabeling is that the substitution of [ 11 C] for a nonradioactive [ 12 C] carbon atom does not alter the amino acid’s chemical characteristics. Certain amino acids have gained more popularity than others as metabolic imaging agents because of differences in relative ease and cost of production, biodistribution, and formation of radiolabeled metabolites. Positron labeled amino acid analogues have in general a low background uptake in gray and white matter, which allows easier detection of tumors (especially small ones).
Whereas the most studied radiolabeled amino acid for PET imaging of brain tumors is MET, other 18 F-labeled aromatic amino acid analogues have been developed recently for tumor imaging including FET and l- 3,4-dihydroxy-6-[ 18 F]fluorophenylalanine (FDOPA), because of the short half-life of [ 11 C] (20 minutes) that requires an on-site cyclotron. The other radiolabeled amino acid PET tracers include l- 1-[ 11 C]tyrosine (TYR), and l- 3-[ 18 F]fluoro-α-methyltyrosine (FMT).
MET-PET
MET-PET has been one of the most popular amino acid imaging modalities in neuro-oncology, although its use is restricted to PET centers with an in-house cyclotron facility. This popularity is largely due to its relative ease of production that lacks complicated purification steps.
The precise underlying molecular mechanisms this methionine dependence remains yet to be completely elucidated. Methionine, a sulfur-containing essential amino acid, is transported mainly through Na + -independent system L (leucine preferring) and Na + -dependent systems A (alanine preferring) and ASC (alanine, serine, cysteine). LAT1 (high substrate affinity), 1 of the 2 membrane-spanning proteins belonging to system L, is strongly expressed in malignant glioma cells, and there is enhanced facilitated transport of amino acids in the supporting vasculature of the tumors due to upregulation of amino acid transporter expression. The rates of transmethylation are frequently increased in human tumor cells, and this can lead to the “methionine dependence” observed in vitro tumor cell lines. Overall, the increased uptake of methionine in cancer cells caused by increased in fluxes of the amino acids (mediated largely by the L system of transport), enhanced the pathways of protein synthesis, transmethylation, and transsulfuration.
MET-PET has been studied to determine its value in a variety of roles in the evaluation of brain neoplasms, including: detection and delineation of tumor, tumor grading, prognosis, and effectiveness of therapy.
Detection and Delineation of Tumor Extent with MET-PET
MET-PET has been demonstrated by multiple studies to be a sensitive tool for detection of brain neoplasms. The overall sensitivity of MET-PET for malignant gliomas (both low and high grades) has been higher compared with FDG-PET ( Fig. 5 ), ranging from 76% to 95%, with higher rates for higher grade neoplasms. In low-grade gliomas, the range of reported sensitivities lies between 65% and 85%. In one study, the utility of MET-PET in 45 brain lesions that were iso- or hypometabolic on FDG-PET was evaluated. MET demonstrated increased uptake in 31 of 35 tumors with 89% sensitivity. For 24 gliomas, MET demonstrated a positive uptake in 22 with a sensitivity of 92%. All 10 benign lesions (cysticercosis, radiation necrosis, tuberculous granuloma, hemangioma, organized infarction, and benign cyst) showed normal or decreased MET uptake (100% specificity), whereas false-negative MET-PET was encountered in intermediate oligodendroglioma, metastatic tumor, chordoma, and cystic ganglioma. As is discussed in greater depth later, increased uptake of MET was also seen in neoplasms in which there was little or absent contrast enhancement on MR imaging and low uptake on FDG-PET, findings that are typically noted in the low-grade gliomas.