This article reviews current amyloid positron emission tomography (PET) imaging with particular attention to Pittsburgh compound-B (PiB), the most extensively investigated and validated tracer. PiB specifically binds to fibrillar β-amyloid deposits such as those found in the cerebral cortex and striatum. PiB-PET imaging is a sensitive and specific biologic marker for underlying amyloid deposition, which is an early event on the path to dementia. Amyloid imaging in healthy controls and patients with mild cognitive impairment may detect those at high risk of future Alzheimer’s disease, identifying them as candidates for early preventive measures if and when they become available.
Dementia is a serious loss of cognitive ability in a previously unimpaired person beyond what might be expected from normal aging. Dementia that begins gradually and worsens progressively over several years is usually caused by neurodegenerative disease, that is, by conditions affecting only or primarily the neurons of the brain and causing gradual but irreversible loss of function of these cells. Worldwide increases in the number of people with dementia, the highest proportion of whom are affected by Alzheimer’s disease (AD), have made its early diagnosis a major research and clinical priority. The cause of AD is unknown, but typical changes in the brain are neuronal loss, numerous globs of sticky proteins (β-amyloid [Aβ] plaques) in the spaces between neurons, a tangled bundle of fibrils within neurons (neurofibrillary tangles). Although it is still unclear what the relationship is between amyloid pathology and neuronal degeneration, progressive neuronal loss, and subsequent atrophy of various cortical gray matter structures, the most prominent hypothesis for the cause of AD remains the amyloid cascade hypothesis, which holds that the Aβ peptide is the key to the initiation and progression of the disease. Pathologic studies are insufficient to validate this theory, because they are always inevitably cross-sectional and cannot determine how key events are temporally related to each other. During the past several years, amyloid imaging has established itself alongside magnetic resonance (MR) imaging and fluorodeoxyglucose (FDG)-positron emission tomography (PET) as a surrogate marker for the investigation of brain aging and dementia.
Functional neuroimaging, such as FDG-PET and brain perfusion single-photon emission computed tomography (SPECT), has been widely used for imaging biomarkers of AD. Recent advances in instruments have facilitated investigations of functional alterations in fine structures of not only cortical but also subcortical areas with high spatial resolution. Metabolic and perfusion reductions in the parietotemporal association cortex are recognized as a diagnostic pattern for AD. Outstanding progress in the diagnostic accuracy of these modalities has been achieved using statistical analysis on a voxel-by-voxel basis after anatomic standardization of individual scans to a standardized brain volume template instead of visual inspection or a volume-of-interest technique. In a very early stage of AD, this statistical approach revealed hypometabolism or hypoperfusion in the posterior cingulate cortex and precuneus. In some countries where FDG-PET has not yet been accepted for reimbursement for the detection of dementia in the health insurance system, more widely available brain perfusion SPECT has been used for the imaging diagnosis of AD. FDG-PET is superior to SPECT in diagnosing early AD because of its higher sensitivity and higher spatial resolution, and FDG-PET offers many advantages for detecting abnormalities in the AD brain. SPECT offers the advantages of lower cost and ease of access, which could lead to a large increase in the number of cases studied using this technique. Although FDG-PET shows more robust separation of patients with AD from healthy volunteers than SPECT, good correspondence of changes in the parietotemporal and posterior cingulate cortices and precuneus in mild to moderate AD is observed using voxel-based statistical image analysis between FDG-PET and SPECT.
Amyloid imaging allows for more rigorous quantification of the distribution and burden of amyloid, and enables direct comparison of these measures with simultaneously derived cognitive metrics in contrast to the significant delay between behavioral assessment and autopsy often seen in postmortem studies.
Among several compounds that have been developed for the imaging of amyloid, N -methyl-[ 11 C]2-(4′-methyl-aminophenyl)-6-hydroxybezothiazole or simply Pittsburgh compound-B (PiB) is a derivative of the amyloid-binding dye thioflavin T and the most extensively validated tracer. It binds to aggregated, fibrillar Aβ deposits, such as those found in the cerebral cortex and striatum, but not to the amorphous Aβ deposits, such as those that predominate in the cerebellum. The first PiB-PET study in humans was performed in patients with mild AD, in whom the uptake pattern was consistent with Aβ plaque deposition described in postmortem studies of AD brains. Postmortem studies of patients who showed increased PiB deposition during life showed high correlations between in vivo PiB accumulation and in vitro measures of Aβ pathology. This article reviews the rapidly expanding literature applying PiB-PET to study cognitively normal volunteers and patients with mild cognitive impairment (MCI) and AD and summarizes the contribution of PiB-PET in understanding the association between amyloid plaques, aging, and dementia.
PiB-PET image analysis
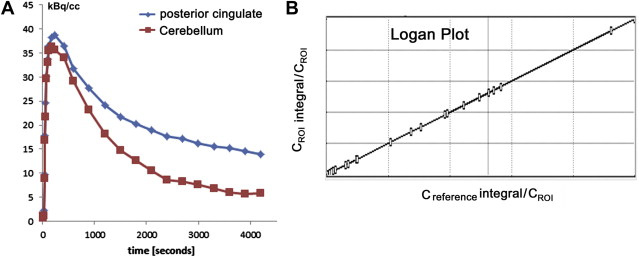
Although visual reading of PiB images seems more accurate than that of FDG for identification of AD, better accuracy is obtained using a quantitative approach without requiring the expertise of readers. PiB binding to Aβ plaque in the gray matter is specific and reversible, whereas PiB binding in the white matter is nonspecific and nonsaturable. The relatively slow kinetics of PiB makes the specific PiB uptake in the gray matter prominent at later time points, which may impede the quantification of Aβ deposits because of the short half-life of 11 C-labeled tracer. To overcome this drawback in quantification, three-dimensional dynamic sampling of emission data for the whole brain is desirable, lasting 70 to 90 minutes after tracer injection. Application of the linear models developed by Logan to these sampling data has become a standard calculation method for robust quantification in PiB studies ( Fig. 1 ). Logan analysis is used to calculate the distribution volume of ligand tracers that have reversible binding kinetics. The choice of cerebellar cortex as a reference region that has no specific PiB deposition enables calculation of a distribution volume ratio (DVR) without arterial plasma data sampling as a slope of a graphical plot. The DVR equals binding potential + 1. The pons can also be chosen as a reference area. On the other hand, the standardized uptake value ratio (SUVR) has been proposed as a more feasible semiquantitative analysis than DVR ( Fig. 2 ). SUVR is calculated by computing the region/cerebellum ratio at later time points. The optimal time range was studied by McNamee and colleagues ; their suggestion was to use a 40- to 60-minute period in studies limited by low injected dose, or a 50- to 70-minute period because of greater measurement stability, especially for longitudinal multisite studies ( Fig. 3 ). The advantages of the SUVR approach are large effect sizes for AD and control group differences, and the possibility of obtaining the required data from a single short scan of 20 minutes. The disadvantages of this approach are the lower test-retest variability compared with the DVR approach and the potential for time-varying outcomes. It also has the inherent bias of a tendency to overestimate PiB deposition. The use of a standardized volume-of-interest template with spatially normalized PiB images to the same standardized space has been proposed as an automated voxel-based method for PiB deposition analysis.
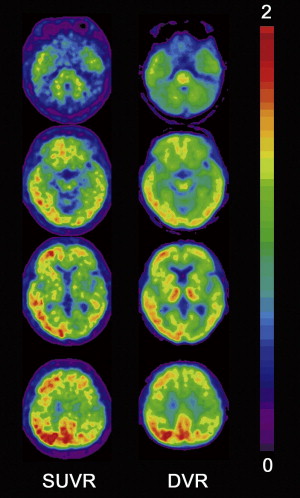
Regions that share a border with lower-binding or higher-binding structures are susceptible to partial volume effects because of a blurring caused by the low resolution of PET. Because gray matter, white matter, and cerebrospinal fluid (CSF) have different PiB uptake patterns, all gray matter boarders undergo partial volume effects. Atrophy of a region that increases the amount of neighboring CSF accentuates these partial volume effects. Applying partial volume correction to PiB-PET has been expected to increase the PiB deposition in atrophied gray matter and lead to more accurate quantification. Partial volume correction is usually performed using segmented gray matter from three-dimensional MR imaging coregistered to PiB images.
PiB-PET in normal controls
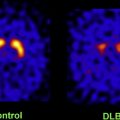
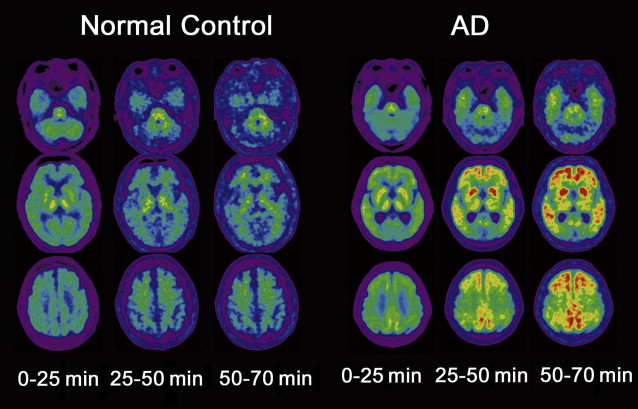
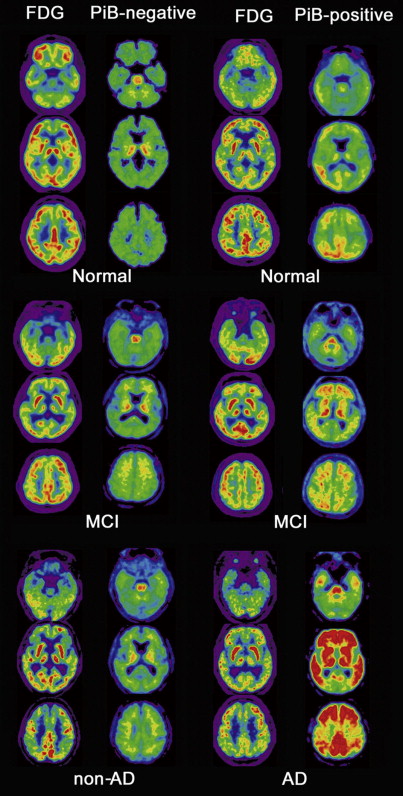
Several autopsy studies have reported that significant Aβ deposits can be found post mortem in more than 30% of cognitively normal older individuals and that the extent of Aβ pathology may be indistinguishable from that found in AD. In accordance with these autopsy results, several PiB-PET studies have consistently detected increased PiB binding in a subset of normal older volunteers ( Fig. 4 ), with the proportion of PiB-positive cases ranging from 10% to 30% depending on the age of the cohort and the threshold for defining PiB positivity. In contrast, increased binding has not been reported in young normal controls. Several studies suggest preferential PiB deposition in the prefrontal cortex and posterior cingulate/precuneus similar to the regions of earliest Aβ deposition noted in autopsy studies. Some older controls show a distribution pattern of PiB binding that is essentially indistinguishable from that seen in AD. The high rate of PiB positivity in normal controls suggests that a positive PiB scan cannot be interpreted without a careful clinical evaluation and emphasizes that amyloid imaging alone must not serve as a surrogate for a clinical diagnosis of AD.
The most important risk factors for AD are age, family history, and heredity. The relationship between these risk factors and PiB deposition has been investigated using a voxel-based statistical analysis. Advancing age increases PiB-positive frequency in normal controls: 18% in those aged 60 to 69 years, rising to 65% in those older than 80 years. The prevalence of Aβ deposition, as detected post mortem in cognitively normal subjects, exponentially increases with advancing age. The prevalence of PiB-positive normal controls increases with advancing age in a similar exponential fashion but precedes the postmortem study by 10 to 15 years. Aβ deposition seems almost inevitable with advancing age. A recent study shows that normal controls with a parent affected by late-onset AD have increased PiB deposition in brain regions typically affected in patients with clinical AD compared with normal controls with no family history. In addition, significant parent-of-origin effects on Aβ deposition were found. Normal controls with mothers affected by late-onset AD show increased and more widespread PiB deposition than those with affected fathers. Another recent study showed that PiB deposition in normal controls correlates with apolipoprotein E (APOE) ε4 gene dose, which is the best known genetic risk factor for AD. The APOE4 allele increases the risk of the disease by 3 times in heterozygous individuals and 15 times in homozygotes. Emerging evidence suggests there may be other risk factors for AD. Epidemiologic studies suggest that cardiovascular risk factors such as increased blood pressure in midlife are associated with increased risk of AD in late life. Langbaum and colleagues revealed that systolic blood pressure and pulse pressure were both positively correlated with PiB depositions. These preliminary findings provide additional evidence that higher BP, which is likely to reflect arterial stiffness during late midlife, may be associated with increased risk of presymptomatic AD. There is some evidence that only angiotensin receptor blockers and angiotensin-converting enzyme inhibitors, and not other blood-pressure–lowering medications, are associated with reduced risk of AD. Antihypertensive treatments may protect against AD neuropathology, potentially by reducing vascular and arterial stiffness, thereby increasing blood flow to the brain and aiding in the removal of Aβ.
A major unresolved issue in AD research is whether cognitively normal people with amyloid deposition are on a trajectory toward AD, or whether the disease is benign in these individuals. Cross-sectional studies evaluating the influence of PiB binding on brain structure and cognition in older control individuals have yielded seemingly conflicting results. When individuals are dichotomized into PiB-positive and PiB-negative groups, most studies have not found significant differences in cognitive performance, with the exception of a study that found lower episodic memory scores in the PiB-positive group. In contrast, most studies that evaluated PiB deposition as a continuous variable have found significant negative correlations between PiB uptake and episodic memory scores. Mormino and colleagues revealed significant correlations between PiB deposition and episodic memory, PiB deposition and hippocampal volume, as well as hippocampal volume and episodic memory. This observation suggests that declining episodic memory in older individuals may be caused by Aβ-induced hippocampal atrophy. Becker and colleagues found the significant Aβ-associated cortical thinning particularly in parietal and posterior cingulate regions extending into the precuneus in a pattern consistent with early AD among nondemented older individuals. This finding suggests that Aβ-associated neurodegeneration is manifest as cortical thinning in regions vulnerable to early Aβ deposition. Oh and colleagues found that gray matter volume in the left inferior frontal cortex was negatively associated with amyloid deposition across all participants of nondemented older controls, whereas reduced gray matter volume was shown in the posterior cingulate among older controls with high amyloid deposition. This reduction of gray matter volume in the left inferior frontal cortex was associated with poorer working memory performance. The pattern of Aβ deposition as detected by PiB-PET shows substantial spatial overlap with the default mode network comprising a group of brain regions that typically deactivate during externally driven cognitive tasks. Functional connectivity in the default mode network is altered with increasing levels of PiB uptake. These findings highlight structural and cognitive changes in association with the level of Aβ deposition in cognitively intact normal elderly individuals.
Longitudinal studies evaluating the relationship between PiB deposition and cognitive decline or brain atrophy have also yielded conflicting results. Dricoll and colleagues examined associations between PiB deposition and brain volume changes in the preceding years in 57 nondemented individuals. Despite significant longitudinal decline in the volumes of all the regions investigated, no associations were detected between PiB deposition and regional brain volume decline trajectories in the preceding year, nor did the regional volume trajectories differ between those with highest and lowest Aβ burden. These findings suggest that Aβ load does not seem to affect brain volume changes in individuals without dementia. These investigators’ observations are in agreement with existing reports on PiB deposition and brain volume loss. Jack and colleagues investigated MR imaging and PiB studies at 2 time points, approximately 1 year apart, to gain insight into the sequence of pathologic events in AD. These investigators reported a dissociation between the rate of amyloid deposition and the rate of neurodegeneration late in life over 1 year of follow-up. Amyloid deposition proceeded at a constant low rate irrespective of clinical status, whereas neurodegeneration accelerated in association with clinical symptoms. These findings suggest that in nondemented elderly individuals, amyloid accumulation does not affect the rate of brain atrophy beyond that already observed as a part of the normal aging process. On the contrary, Villemagne and colleagues reported that normal controls who progressed to MCI or AD over 38 months had significantly lower memory scores, higher baseline, and greater increase of PiB deposition than those normal controls who did not progress. Sojkova and colleagues also found that longitudinal increases in Aβ deposition varied among individuals. This variability in the annual rate of change was affected by PiB deposition at the initial PET study, and increases were greater in nondemented older adults with an increased Aβ level compared with a minimal Aβ level at the initial evaluation. Furthermore a longitudinal cohort study was performed to determine whether preclinical AD, as detected by PiB-PET in cognitively normal older adults, is associated with a risk of symptomatic AD. Twenty-three of 159 participants with a clinical dementia rating (CDR) of 0 progressed to CDR 0.5 at follow-up assessment. More increased PiB deposition highly predicted progression to CDR 0.5, with a hazard ratio of 4.85. These findings suggest that PiB deposition is not benign, because it is associated with progression to symptomatic AD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree