Greater trochanteric syndrome (GTS) is a common condition clinically manifested by pain and tenderness over the greater trochanter. MR imaging plays a pivotal role in investigating the underlying cause of GTS. MR imaging can detect abnormalities not only in symptomatic but also in asymptomatic hips, thereby revealing structural damage in the gluteal tendons and muscles during both clinical and preclinical phases. This review article emphasizes the importance of detailed knowledge of anatomy of the greater trochanter and adjacent soft tissues, along with the imaging appearance of pathology of the abductor tendons and muscles as well as the peritrochanteric bursae.
Key points
- •
Greater trochanteric syndrome (GTS) is a common condition clinically manifested by pain and tenderness over the greater trochanter.
- •
GTS is usually a combination of pathologies involving the gluteus minimus (GMi) and gluteus medius (GMe) tendons, peritrochanteric bursae, and the lateral myofascial sheath of the hip.
- •
MR imaging is a promising biomarker for diagnosing GTS, as it can detect abnormalities in the GMi and GMe tendons and other peritrochanteric tissues in clinical and preclinical phases.
- •
MR imaging is both sensitive and specific in detecting structural damage in the gluteal tendons and muscles, ultimately predicting mechanical dysfunction.
Introduction
Improvements in visualization of the extra-articular tissues of the hip by arthroscopy and advanced imaging methods, such as MR imaging, have recently heightened interest in studying the soft tissues surrounding the greater trochanter. , To further motivate research in this area, abnormalities in the peritrochanteric tissues have been implicated in triggering lateral hip pain and gait dysfunction, as well as contributing to a poorer outcome after total hip replacements.
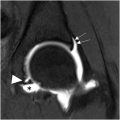
The terminology “greater trochanteric pain syndrome” is currently used to describe chronic lateral hip pain and tenderness over the greater trochanter caused by disorders in the peritrochanteric space, replacing the old terminology “greater trochanteric bursitis” , ( Fig. 1 ). Historically, lateral hip pain has been attributed to trochanteric bursitis (which is a great mimicker of other hip disorders), without further investigation of the underlying cause. , A change in the approach to this clinical presentation emerged after ultrasound and MR imaging studies showed that the condition is usually a combination of pathologies involving the gluteus minimus (GMi) and gluteus medius (GMe) tendons, peritrochanteric bursae, and the lateral myofascial sheath of the hip ( Fig. 2 ). In addition, isolated bursitis is present in only the minority of patients. ,


Greater trochanteric pain syndrome is a common clinical condition found alone or associated with other pathologies that affect the hip and other joints in the kinetic chain. For instance, its incidence has been reported as 1.8 per 1000 patients per year in primary care, while its prevalence has been estimated as 17.6% in adults at risk for knee osteoarthritis and 20% to 35% in adults with low back pain. , , The syndrome has been described as more prevalent in the fourth to sixth decades of life and 4 times higher in females than males, which can aggravate problems related to menopause. Some athletes, such as runners, footballers, and dancers, compose another epidemiologic group with a higher incidence of the syndrome. Despite the epidemiologic differences in risk groups, they share a common mechanism of injury.
MR imaging has been proposed as a promising biomarker for several pathologies in the musculoskeletal system, both in the preclinical and clinical phases. In the hip, MR imaging was able to detect abnormalities in the GMi and GMe tendons and other peritrochanteric tissues not only in symptomatic but also in asymptomatic hips. Hence, in this review article focused on the MR imaging diagnosis, the authors will use the umbrella terminology greater trochanteric syndrome (GTS) rather than greater trochanteric pain syndrome to encompass abnormalities in both symptomatic and asymptomatic hips (see Fig. 1 ). In addition, the authors will emphasize the anatomy of the greater trochanter and adjacent soft tissues for a better understanding and interpretation of normal and pathologic imaging findings associated with GTS.
Mechanism of Injury
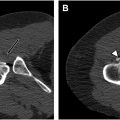
A mechanism of injury proposed for GTS is repetitive friction and compression of the iliotibial band (ITB) and anterior portion of the GMax muscle on the greater trochanter, especially during movements, leading to microtrauma of the interposed soft tissues , ( Fig. 3 ). With overuse, the GMi and GMe tendons are prone to infiltration and tears and the peritrochanteric fat and bursae are prone to reactive edema and inflammation. In addition to overuse, GTS can be triggered by altered gait patterns and localized trauma.

Normal anatomy and imaging technique
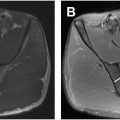
The greater trochanter is a bony prominence at the junction of the femoral neck and shaft that projects superolaterally and extends anteriorly and posteriorly. There are 4 well-defined facets: anterior, lateral, superoposterior, and posterior, with only the latter completely lacking a tendon insertion ( Fig. 4 ). The greater trochanter is the primary site of insertion of the main hip abductors, that is, the GMi and GMe muscles. The tensor fascia latae is another muscle that assists hip abduction but inserts into the ITB instead. The trochanteric facets also have an intimate relationship with several bursae that cushion and reduce friction between the bone and the adjacent soft tissues.

Anterior Facet, Gluteus Minimus, and Subgluteus Minimus Bursa
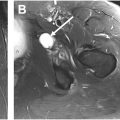
The anterior facet has an oval or slightly concave configuration and lies just lateral to the capsular insertion of the hip , (see Fig. 4 ; Figs. 5 and 6 ). This facet serves as the insertion site of the main tendon of the GMi muscle. The anterior facet is usually best visualized on MR imaging in the axial plane (see Fig. 5 ). However, coronal and sagittal are secondary planes where this facet can also be well seen (see Fig. 6 ; Fig. 7 ). In addition, it is possible to identify the anterior part of the GMe muscle covering the GMi tendon insertion into the anterior facet in the axial and coronal planes (see Figs. 5 and 6 ).



The muscle belly of the GMi is often divided into anterior and posterior parts. However, the distal insertion is usually referred to as long and capsular heads, corresponding to the main tendon of the GMi and the direct insertion of the GMi muscle into the hip capsule, respectively. , On MR imaging, the axial plane is the best plane for characterizing the different parts of the muscle belly and visualization of the main tendon (long head). In contradistinction, the coronal plane is the best to visualize the relationship between the GMi muscle and the joint capsule (capsular head) (see Fig. 6 ).
The subgluteus minimus bursa partially covers the anterior facet medially and has an intimate relationship with the joint capsule and femoral tubercle. The femoral tubercle can be used as an imaging landmark for locating this bursa.
Lateral and Superoposterior Facets, Gluteus Medius, and Subgluteus Medius Bursa
The lateral facet has a trapezoid configuration, with its lower part resembling an inverted triangle. , This facet is unique in that it is surrounded by the other facets of the greater trochanter. A ridge is often seen between the lateral, anterior, and superoposterior facets but is usually lacking between the lateral and posterior facets. In the coronal plane, the facets are visualized on sequential images from anterior to posterior. In the axial plane, the anterior, lateral, and posterior facets can be visualized simultaneously. Due to variations in hip rotation in the MR imaging studies, cross-referencing can be an important tool to facilitate the identification of the facets in the coronal plane.
A bare spot (A.K.A. bald spot) without tendon insertion has been described on the lateral facet between the insertions of the GMi and GMe tendons. , The anterior subgluteus medius bursa lies in this bald area, cushioning the facet of parts of the GMe muscle and tendon. Careful attention should be given to distinguishing the anatomic bald area from the pathologic bald trochanter, the latter representing an uncovered entheseal attachment due to a tear of the GMi and or GMe tendons.
Unlike the GMi muscle, which inserts into just 1 facet (the anterior), the GMe muscle inserts into 2 facets, the lateral and superoposterior. The GMe muscle has been divided anatomically into anterior, middle (central), and posterior parts, which function in a phasic manner during the gait cycle. Most of the lateral facet footprint is determined by a thin and broad insertion of the anterior part of the muscle. The middle part inserts into the lateral facet at the junction with the superoposterior facet. Finally, the posterior part inserts into the superoposterior facet by way of a thick and strong tendon, which is considered the main tendon of the GMe muscle (see Fig. 7 ). ,
The superoposterior facet is the most cephalad portion of the trochanter and is more medially located than the lateral facet. The superoposterior and lateral facets are oriented at differing angles to each other. The lateral and superoposterior facets are well visualized on MR imaging in the coronal and sagittal planes.
Posterior Facet and Trochanteric Bursa
As previously mentioned, the posterior facet lacks tendon insertion and represents another bald area of the greater trochanter. The deep subgluteus maximus bursa, also called “trochanteric bursa (TrB),” lies superficial to it, functioning as a cushion for the pressure of the adjacent GMax muscle and ITB (see Figs. 3 and 4 ). On MR imaging, this facet has a curved contour in the axial plane and a straight contour in the sagittal plane.
Imaging findings
Standard and Advanced Magnetic Resonance Protocols
The standard magnetic resonance (MR) protocol for GTS should incorporate anatomic [T1-weighted and proton density (PD)-weighted sequences] and fluid-sensitive sequences (short tau inversion recovery [STIR] and T2/PD-weighted fat-saturated sequences). These sequences help evaluate bone, muscle volume loss, and fatty infiltration, as well as tendon pathologies such as tendinosis and tendon tears. Additionally, peritrochanteric high signal intensity on fluid-sensitive sequences can be assessed to provide insight into reactive edema and bursitis.
The slice thickness and spacing should be optimized to balance image quality and scan time. Thinner slices (≤3 mm) are typically used for better spatial resolution. Three-dimensional isotropic sequences with submillimeter slice thickness, such as CUBE, are an option for a single rapid acquisition for an entire hip with the advantage of reformats in any imaging plane. One challenge in hip MR imaging is the presence of metal implants, such as those used in hip arthroplasty. They can cause susceptibility artifacts, which distort regional anatomy and degrade the periprosthetic signal. Modifications to routine imaging techniques to mitigate arthroplasty-related artifacts include imaging at 1.5-T instead of 3.0-T, increasing gradient amplitudes, using inversion-recovery fat suppression instead of frequency-selective fat suppression, and employing metal artifact reduction sequences such as multiacquisition with variable resonance image combination and slice-encoding metal artifact correction. ,
Correlation Between MR imaging and Clinical Findings
MR imaging has been considered the imaging modality of choice in investigating GTS due to its high soft tissue contrast, multiplanar capabilities, and strong correlations between imaging interpretation and intraoperative findings. The technique has proven excellent in detecting structural damage in the gluteal tendons and muscles, ultimately predicting mechanical dysfunction. For instance, fatty infiltration of the gluteal muscles has been shown to correlate with poor functional outcomes in patients with total hip arthroplasty. , However, like in other conditions in the musculoskeletal system, MR imaging has been reported to have a poor to moderate correlation with pain in GTS. ,
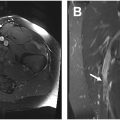
Gluteal Tendon Pathology
Several structural changes occur in tendons with degeneration, such as collagen disorganization, fibrillar structure loss, mucoid material accumulation, increased water, and altered proteoglycan content. These structural changes can be visualized by conventional MR imaging as tendon thickening and areas of increased signal intensity. The distinction between tendinosis and tear has traditionally been made using the signal intensity of the free water (fluid) as a reference ( Figs. 8 and 9 , Table 1 ). The signal intensity of tendinosis has been described as not as high as fluid on fluid-sensitive sequences, as opposed to the signal intensity of the tear, which is isointense to fluid.