MR imaging is the gold standard for diagnosis, providing detailed evaluation of perianal fistulas. MR imaging aids in detecting, classifying, and monitoring fistulas and guiding treatment. Detailed radiology reports, incorporating patient history and disease-specific considerations, are essential for effective management and improved clinical outcomes. This review overviews fundamental high-yield concepts to aid radiologists in interpreting MR imaging for perianal fistulas with multiple case examples.
Key points
- •
MR imaging is the gold standard for the evaluation of perianal fistulas.
- •
Dedicated perianal MR imaging protocols focus on the anorectal anatomy, utilizing small field-of-view imaging, often at 3.0 T, with or without intravenous contrast. The protocol provides high spatial resolution and multiplanar visualization.
- •
Radiology reports should be structured and detailed and integrate clinical history to address disease-specific considerations.
- •
The management and understanding of perianal fistulas continue to evolve and require a multidisciplinary approach.
Introduction
A perianal fistula, also known as a fistula-in ano, is an abnormal, acquired communication between the lumen of the rectum or anal canal and the skin surface of the perineum. Active fistulas have a fibrous wall with internal granulation tissue or fluid, with tracts becoming more fibrous as they heal. Fistulizing perianal disease affects patients of all ages and is a significant cause of morbidity and decreased quality of life in the United States and worldwide. , Treatment of perianal fistulizing disease is challenging and often a lengthy process requiring multiple follow-up visits to evaluate healing and resolution.
Pathogenesis of perianal fistulas is theorized to result from infection or inflammation of glands and crypts within the lumen of the rectum or anal canal. Resultant inflammatory changes surround a mural abscess, and the natural healing process may result in a chronic perianal tract or fistula. Risk factors predisposing patients to fistula formation include inflammatory disorders of the bowel, including Crohn’s disease, as well as a history of perianal trauma (iatrogenic and childbirth), history of pelvic radiation, or comorbidities and lifestyle factors that increase inflammation and impair healing such as diabetes, smoking, obesity, and diet. These are summarized in Box 1 .
Cryptoglandular infection (idiopathic)
Crohn’s disease
Sexually transmitted infection
Chronic diarrhea
Diverticulitis
Radiation
Trauma to the anal area (eg, childbirth)
Anorectal cancer
Tuberculosis
Foreign body
Diagnosis of perianal fistulas has historically been made using a combination of history and physical examination, with local tenderness and pain frequently necessitating examination under anesthesia (EUA). Endoanal ultrasound can also be used to characterize and guide perianal fistula treatment. , The primary drawback to both techniques is that they are operator dependent, and visualization and assessment are limited to the areas immediately adjacent to the observer, either visible on the perineum or anoscope or adjacent to the ultrasound probe, so tracts and abscesses remote from the sphincter complex may be missed. Pelvic MR imaging has emerged as the gold standard for the evaluation of perianal fistulizing disease, offering more operator independence, visualization of more remote areas such as the supralevator space, visual interrogation by all members of the interdisciplinary care team, high spatial resolution and soft tissue contrast, multiplanar visualization of complex branching fistulas and abscesses not detected at EUA, and the opportunity to monitor treatment response or healing and has been shown to decrease the need for surgical intervention.
Perianal MR imaging technique
Dedicated perianal MR protocols differ from general pelvis MR protocols in that they consist of a targeted small field-of-view imaging oriented to the specific anatomy of the anorectum. Protocols vary between institutions, and imaging may be performed at 1.5 or 3.0 T, with or without intravenous (IV) contrast. Phased array surface coils can be used to maximize image quality. A typical perianal MR protocol is shown in Table 1 . In the authors’ experience, IV contrast is helpful, and the increased signal-to-noise ratio and spatial resolution afforded at 3.0T are beneficial for small field-of-view imaging when available. No specific patient instructions are required before the examination, and antiperistaltic drugs and bowel preparation, such as enemas or pre exam fasting status, are typically not needed.
| Plane | Weighting | FOV | Coverage | Special Notes |
|---|---|---|---|---|
| Three plane scout | Centered on pelvis | |||
| Sagittal oblique | T2 | 22 cm | Between hip joints left to right, include the entire perineum | |
| Axial oblique | T2 | 22 cm | Oriented to the anal canal Acetabular roof through perineum/buttocks | Fat saturated |
| Axial oblique | T2 | 22 cm | Oriented to the anal canal Acetabular roof through perineum/buttocks | |
| Coronal oblique | T2 | 22 cm | Oriented to the anal canal Anterior sacrum to posterior pubic symphysis SI FOV acetabular roof through perineum buttocks | |
| Axial oblique | T1 (Spoiled Gradient Echo) | 28 cm | Oriented to the anal canal Acetabular roof through perineum/buttocks | Post-contrast fat saturated |
| Sagittal oblique | T1 | 28 cm | Between hip joints left to right, include the entire perineum | Post-contrast fat saturated |
| Coronal oblique | T1 | 28 cm | Oriented to the anal canal Anterior sacrum to posterior pubic symphysis SI field of view (FOV) acetabular roof through perineum buttocks | Post-contrast fat saturated |
| Optional Sequences | ||||
| Axial | T1 | 30+ cm | FOV to cover the entire pelvis depending on patient size | Post-contrast fat saturated |
| Axial oblique | T1 | 22 cm | Oriented to the anal canal. Acetabular roof through perineum/buttocks | Pre-contrast without fat saturation |
| Axial oblique | DWI | 22 cm | Oriented to anal canal. Acetabular roof through perineum/buttocks | |
Normal anatomy
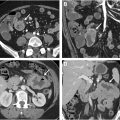
The anal sphincter complex comprises the distal 4 to 5 cm of the gastrointestinal tract ( Fig. 1 ). The 2 primary muscles that form the anal sphincter complex are the internal and external anal sphincters. The internal anal sphincter is made up of smooth muscle fibers oriented circumferentially and is contiguous with the rectal smooth muscle superiorly. The external anal sphincter is a trilaminar structure made up of skeletal muscle fibers. The external anal sphincter can be further subdivided into 3 parts: the deep part, the superficial part, and the subcutaneous part. The deep external anal sphincter comprises circular muscle fibers, and the superior margin is contiguous with the puborectalis of the levator ani muscle group. The superficial external anal sphincter demonstrates elliptical muscle fibers that attach to the coccyx posteriorly and the perineal body anteriorly. The subcutaneous external anal sphincter again has circular muscle fibers and serves as the inferior portion of the sphincter complex. Based on the dual structure of the sphincter complex with smooth and skeletal muscle, innervation of the autonomic component comes from sympathetic and parasympathetic fibers of the superior/inferior hypogastric plexus. In contrast, the somatic component is fed via pudendal nerve branches. MR imaging appearance of the anal sphincter complex is shown in Fig. 2 A–C.


Classification systems
Preoperative evaluation with MR imaging has been shown to accurately classify perianal fistulas, reducing the recurrence rate following surgery. The accuracy of pelvic MR imaging for perianal fistulas ranges from 86% to 100%. , Perianal fistulas have an internal opening in the anus or low rectum and most often extend to an external opening at the skin surface. They can, however, communicate with other epithelialized structures or end blindly, in which case they are considered a sinus tract. The classification of perianal fistulas is based on the relationship between the primary tract and the muscles of the anal sphincter. Two classification systems for perianal fistulas often used in clinical practice include Parks and St. James’ University Hospital classifications.
Parks Classification
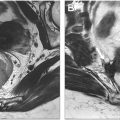
The initial classification of perianal fistulas was published in 1976 and based on an analysis of surgical anatomy in 400 patients referred to St. Mark’s Hospital in London and treated over 15 years. Parks and colleagues described perianal fistulas in the coronal plane according to the course of the fistula and its relationships to the anal sphincter complex. Fistulas were classified into 4 groups: intersphincteric, transsphincteric, suprasphincteric, and extrasphincteric. An additional category, superficial perianal fistulas, is often added to describe fistulas that originate in the low anal canal, which does not traverse muscles of the sphincter complex. Parks classification of perianal fistulas is summarized in Fig. 3 .

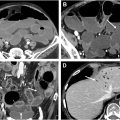
Intersphincteric fistulas (45% of cases in the study) are the most common of the 4 subtypes. The fistula tract runs in the intersphincteric space along the fibers of the internal anal longitudinal muscle between the internal and external sphincters but spares the external sphincter ( Fig. 4 A–C).

In transsphincteric fistulas (30% of cases), the tract passes through the internal anal sphincter, the intersphincteric space, and the external sphincter into the ischioanal fossa and then to the skin ( Fig. 5 A–C ).

Suprasphincteric fistulas (20% of cases) have an internal opening in the mid-anal canal with the fistula tract extending superiorly within the intersphincteric space to a point above the level of the puborectalis and levator ani muscles to enter the supralevator compartment. The fistula then courses inferiorly, penetrates the levator ani muscle to enter the ischioanal fossa, and extends to the skin surface. Suprasphincteric fistulas commonly spread within the supralevator space, and the superior extension may form a “horseshoe”-shaped tract around the rectum ( Fig. 6 A–C).

Extrasphincteric fistulas were the least frequent (5% of cases). They have an internal opening in the low rectal wall. The fistula tract crosses the levator ani muscle to enter the ischioanal fossa and then extends to the skin surface in the perineum ( Fig. 7 A–C). The tract lies outside of the anal sphincter complex.

St. James’ University Hospital Classification
Morris and colleagues proposed the modified classification system, the St. James’ University Hospital Classification. This classification system is based on anatomic landmarks similar to the Parks system but also incorporates abscess formation and secondary ramifications into the scheme. The 5 grades of perianal fistula according to the St. James’ University hospital classification are summarized in the following:
Grade 1: Simple linear intersphincteric fistula —A Grade 1 fistula arises from the anal canal, penetrates through the internal anal sphincter, and extends through the intersphincteric space to the skin of the perineum. There is no involvement of the external anal sphincter and any secondary tract or associated abscess.
Grade 2: Intersphincteric fistula with abscess or secondary tract —A Grade 2 fistula consists of a primary tract and secondary tract(s) or abscess(es) occurring in the intersphincteric space and confined by the external sphincter. This category includes intersphincteric horseshoeing, which refers to secondary fistulous ramifications that extend bidirectionally in the intersphincteric space to wrap around the internal sphincter.
Grade 3: Transsphincteric fistula —A Grade 3 fistula arises from the anal canal, often at the level of the dentate line, and penetrates both the internal and external anal sphincters to cross into the ischioanal fossae before reaching the perineal skin.
Grade 4: Transsphincteric fistula with abscess or secondary tract within the ischiorectal or ischioanal fossa —A Grade 4 fistula is a transphincteric fistula complicated by secondary tract(s) or abscess(es). An abscess may occur anywhere along the primary tract or its ramifications or within or along muscles of the anal sphincter complex.
Grade 5: Supralevator and translevator disease —Grade 5 fistulas include simple or complex (ie, with branching ramifications or abscess) fistulas with supralevator and translevator extension (ie, suprasphincteric or extrasphincteric).
Systematic Approach and Reporting of Perianal Fistula on MR Imaging
Due to their complexity, radiologists should consider a structured and systematic approach when evaluating perianal fistulas. Active inflammation is typically most conspicuous on T2 fat-saturated sequences, and thus, this sequence is well suited for initial detection. On these sequences, the linear hyperintense tracts can be identified and followed to their origin/internal opening at the anal canal. Axial T2-weighted images without fat saturation provide excellent contrast resolution of the anal sphincter complex and allow for better assessment of the anatomic classification of the fistula. Coronal and sagittal images are ideal for detecting supralevator and extra-sphincteric extensions since the relationship between the levator plate and pelvic structures is well delineated. Granulation tissue and fluid/pus within a fistula will both be T2 hyperintense; however, gadolinium-enhanced fat-suppressed T1-weighted images can help distinguish between these findings and a fluid filled tract. Actively inflamed fistulas are often associated with soft tissue edema and inflammatory changes. Skin thickening may be presented adjacent to the external opening.
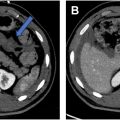
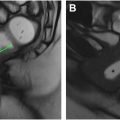
When describing fistulas in the radiology report, it is important to include the location of any internal openings, the anatomy of the fistula, the presence of any secondary fistulas or abscesses, and any supralevator extension. The location of the internal opening(s) should be described using left-right, anterior–posterior descriptors, and/or using the anal clock with the anterior midline representing the 12 o’clock position. Using both types of descriptors provides the clearest communication. The distance of the internal opening from the anal verge should be reported as this distance may be helpful during examination under anesthesia and can also inform treatment planning. Simple fistulas (no branching or associated abscess) should be classified according to Park’s system. If a complex fistula is present, radiologists should classify the primary fistula tract and then separately describe the ramifications that arise from it. Bidirectional horseshoe ramifications typically occur in the intersphincteric space, along the puborectalis muscles, or about the low rectum. Any peripherally enhancing fluid collection measuring greater than 3 mm is considered an abscess. However, abscesses may also contain gas or stool, both of which can result insusceptibility artifacts and signal loss ( Fig. 8 A, B). Maximum tract diameter should be measured (mm) as this can be a useful data point when assessing response. It is particularly important to report supralevator extension as this may not be readily assessed at examination under anesthesia. Lastly, in addition to the fistula itself, the presence or absence of proctitis should be documented and potentially relevant osseous abnormalities (eg, avascular necrosis of the femoral heads, sacroiliitis) should be mentioned.