Spinal vascular lesions are an uncommon but important etiology for myelopathy. A high index of suspicion and appropriate protocol development are critical to correctly diagnose and direct management of these lesions. Contrast enhanced MR angiography should be considered for characterization of these lesions following anatomic image acquisition. When reporting on these cases, the presence of intramedullary spinal cord edema and blood products as well as potential arterial supply and venous drainage should be included.
Key points
- •
Imaging of spinal vascular lesions can require multiple modalities; however, MR imaging is critical in the diagnosis and characterization.
- •
Inclusion of blood sensitive sequences is important to evaluate spinal vascular lesions and subsequent hemorrhage.
- •
Knowledge of the vascular supply to the spinal cord is critical to help guide potential endovascular interventions.
- •
Arteriovenous fistulas often present with slowly progressive myelopathy due to venous hypertension and arteriovenous malformations typically present more acutely from hemorrhage or spinal cord infarcts.
| ASA | anterior spinal artery |
| AVF | arteriovenous fistula |
| AVM | arteriovenous malformation |
| CTA | computed tomographic angiography |
| dAVF | dural arteriovenous fistula |
| DSA | digital subtraction angiography |
| GRE | gradient recalled echo |
| ISSVA | International Society for the Study of Vascular Anomalies |
| MRA | MR angiography |
| PSA | posterior spinal artery |
| STIR | short-tau inversion recovery |
Introduction
MR imaging plays a crucial role in both the detection and characterization of spinal vascular lesions. It is often the first imaging modality to detect these pathologies and trigger targeted workup. In many instances, radiologists are the first to raise the possibility of these lesions, as often advanced imaging is performed due to nonspecific symptoms such as pain or myelopathy. MR imaging often identifies cord edema and potential flow voids within and adjacent to the cord. Knowledge of the role of MR imaging and spinal vascular anatomy is crucial to both identify the abnormality and guide further treatment.
Arterial and venous anatomy of spinal canal
Understanding spinal vascular anatomy and its relationship to the spaces of the spinal canal is crucial to the diagnosis and characterization of these lesions. The spinal cord is surrounding by 3 meningeal layers (from internal to external): pia mater, arachnoid mater, and dura mater. The subarachnoid space is between the pia and arachnoid mater and contains cerebrospinal fluid. The subdural space is between the arachnoid and dura mater. The epidural space is external to the dura mater and contains fat, arteries, veins, and lymphatics.
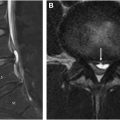
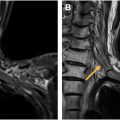
The arterial vascular supply ( Fig. 1 ) to the spinal cord is from a single anterior spinal artery (ASA) running in the anterior median sulcus and paired posterior spinal arteries (PSAs) running along the posterolateral spinal cord.

The ASA supplies the anterior two-thirds of the spinal cord and originates from the intradural vertebral arteries and caudally from segmental arteries, including the ascending and deep cervical, intercostal, lumbar, and sacral arteries. The segmental arteries further subdivide into the radicular arteries which supply the dura/nerve roots (radiculomeningeal branches) and spinal cord (radiculomedullary and radiculopial branches). The dominant radiculomedullary artery, known as the artery of Adamkiewicz , typically arises from a left-sided segmental branch between T8 and L1 forming its characteristic “hairpin” loop joining the anterior spinal artery. Localization of the dominant radiculomedullary artery on noninvasive angiographic imaging is helpful prior to catheter angiography to optimize patient safety (to avoid nontarget embolization with potential ischemic insult to the cord). The paired PSAs supply the posterior one-third of the spinal cord and arise from the posterior inferior cerebellar or intradural vertebral arteries and caudally from segmental arteries, like the ASA. The PSA and ASA anastomose in a pial plexus known as the arterial vasocorona surrounding the spinal cord.
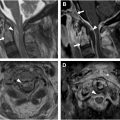
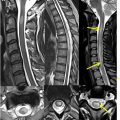
Venous drainage ( Fig. 2 ) of the spinal cord is both complex and variable. Spinal cord drainage is primarily via both intramedullary and extramedullary venous networks including a single anterior and paired posterior spinal veins. These feed radicular branches pierce the dura and enter the vertebral venous plexus including the internal/external venous plexi and basivertebral veins.

Imaging protocols
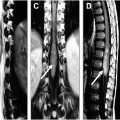
MR imaging is the gold standard imaging modality for evaluating spinal cord pathologies. For shunting spinal vascular lesions, key imaging findings include identification of cord signal abnormality and prominent intradural vascular structures. Importantly, MR imaging allows for detection and aids in localization of spinal vascular lesions but digital subtraction angiography (DSA) is required for further characterization.
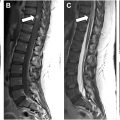
Standard spine MR sequences, including T2-weighted 2-dimensional fast spin echo and short-tau inversion recovery (STIR) sequences, are typically sufficient for detecting cord signal abnormality. Axial gradient recalled echo (GRE) images allows for gray-white matter delineation in the cervical spine and detection of intramedullary spinal cord hemorrhage. Post-gadolinium imaging also helps discriminate nonspinal vascular lesion pathologies, namely spinal cord neoplasms and inflammatory etiologies. The presence of dilated intradural vascular structures is variable for non-dural arteriovenous fistula (dAVF) shunting lesions; however, if cord compression is present from dilated intradural vascular structures, this is suggestive of a non-dAVF shunt lesion. Importantly, the location of spinal cord signal abnormality and abnormal flow voids do not correlate with the location of the arteriovenous fistula (AVF) or arteriovenous malformation (AVM).
Advanced MR techniques can provide further characterization following conventional spinal MR imaging and importantly streamline catheter angiography. Isotropic or 3-dimensional steady-state gradient echo T2-weighted imaging (eg, Siemens, CISS; General Electric [GE], FIESTA) can provide increased spatial resolution aiding in the identification of vascular structures. However, the intrinsically lower soft tissue contrast limits evaluation of cord signal abnormality. Diffusion weighted imaging has a short acquisition time and can aid in the detection of spinal cord ischemic insults.
Contrast-enhanced MR angiography (MRA) using time-resolved techniques (eg, Siemens, TWIST; GE, TRICKS) allows for further characterization of the underlying pathology and targeted catheter angiography. One study showed time-resolved MRA correctly localized dAVFs in over 80% of cases. At this time, time resolved MRA is not sufficient as a screening examination to rule out an arteriovenous shunting spinal vascular lesion. At our institution, these are performed in both the sagittal and coronal planes with 5 mL gadolinium contrast agent injected at 2 mL per second for each acquisition with an optimal temporal resolution of 2 seconds. A suggested vascular protocol is listed in Table 1 . Optimizing time-resolved MRA imaging requires balancing spatial and temporal resolution, which can be challenging when attempting to localize a spinal canal vascular malformation that may have arterial supply from a number of different vessels. Consideration of split dose or double dose MRA with separate upper and lower acquisitions may be helpful, particularly in the thoracic spine.
| MR Imaging Sequences | Advantage | |
|---|---|---|
| Example Standard Protocol | Sagittal: T1 −/+ gad, T2 and T2 FS/STIR Axial: T1 −/+ gad, T2 | Evaluation of the spinal cord for T2 hyperintensity Visualization of intradural flow voids |
| Screening Vascular Protocol—Vascular Images | Contrast-enhanced time resolved MRA Obtained in sagittal and coronal planes. Greater than or equal to 2 years old: 5 mL gadolinium contrast agent injected for each acquisition at 2 mL/s <2 years old: 0.05 mmol/kg gadolinium contrast agent per injection (total = 0.1 mmol/kg) injected at 2 mL/s | Lesion level identification with characterization of feeding arteries and draining veins |
| Screening Vascular Protocol—Anatomic Images | Three-dimensional steady-state gradient echo T2WI Diffuse weighted imaging | Vascular structure identification Spinal cord ischemic insult |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree