This article presents a comprehensive review of MR imaging findings of the sciatic nerve from the pelvis to the knee, highlighting key clinical and diagnostic features of various compressive, neoplastic, and traumatic pathology. Magnetic resonance neurography (MRN) provides an advanced, noninvasive modality for evaluating the nerve anatomy, and its relationship with surrounding structures, enabling precise diagnosis and treatment planning. While high-resolution conventional 2-dimensional sequences such as T1-weighted and T2-weighted fast-spine echo form the backbone of MRN protocols, other 3-dimensional and functional MR imaging techniques can play an important role in facilitating presurgical planning and enhancing more complete tumor characterization.
Key points
- •
Magnetic resonance neurography provides high-resolution imaging of the sciatic nerve to diagnose neuropathies, injuries, and complications, aiding treatment planning and improving patient outcomes.
- •
While still controversial, anatomic variations in the sciatic nerve’s relationship with the piriformis muscle may predispose individuals to conditions like piriformis syndrome or deep gluteal space entrapment.
- •
Nerve compression at various sites is detected on MR Neurography by changes in nerve signal, size, muscle denervation, and diffusion metrics, helping diagnose pathology and assess compression severity.
- •
Neoplastic involvement may be intrinsic or extrinsic; classic MR imaging features like the “target sign” and DWI (with ADC mapping) help differentiate benign from malignant lesions.
- •
Classification systems like Neuropathy Score Reporting and Data System, which generally aligns with Sunderland’s grading of nerve injuries, aim to standardize sciatic nerve injury characterization.
| ADC | apparent diffusion coefficient |
| CIDP | chronic inflammatory demyelinating polyradiculoneuropathy |
| CMT | Charcot–Marie–Tooth |
| CPN | common peroneal nerve |
| DNL | distinct nodular lesion |
| DTI | diffusion tensor imaging |
| DWI | diffusion-weighted imaging |
| FA | fractional anisotropy |
| MRN | magnetic resonance neurography |
| NS-RADS | Neuropathy Score Reporting and Data System |
| WHO | World Health Organization |
| 2D | 2-dimensional |
| 3D | 3-dimensional |
Introduction
Sciatica, or pain radiating down the lower back through the buttocks, into the thigh and lower leg, is a significant cause of patient morbidity. Large survey data ( n >1 million) has shown that up to 25% of Medicare patients report sciatica, imposing large burdens on the health care system. Patients with sciatica also have a higher number of medical comorbidities and score lower on health-related quality of life measures. While about 90% of sciatica has been estimated to be discogenic, a wide spectrum of extraspinal etiologies affecting the sciatic nerve frequently prompt radiological evaluation.
In this case-based review, general principles of MR imaging technique for sciatic nerve imaging and features of sciatic nerve anatomy that predispose to compression neuropathy are elucidated. Emphasized are strategies to differentiate between benign and malignant neoplasms, as well as imaging manifestations of inflammatory etiologies, and grading of sciatic nerve traumatic injuries. Imaging findings after nerve repair and treatment-related complications are shown. It is hoped that this article will provide readers with a better appreciation of how MR imaging can aid in diagnosing and managing the various conditions and pathologies that may affect the sciatic nerve.
Anatomy
The sciatic nerve is the largest nerve in the human body, originating from the anterior and posterior divisions of the anterior rami of L4-S3 spinal nerves, as a continuation of the lumbosacral plexus. The lumbar roots unite anterior to the sacroiliac joint, while the sacral roots unite in front of the piriformis muscle. The sciatic nerve has both sensory and motor functions, exiting the greater sciatic notch as the most lateral structure, deep to the piriformis muscle (variant anatomy discussed later). Medial to the sciatic nerve run the inferior gluteal nerve and vessels, internal pudendal vessels, and pudendal nerve. The tibial and peroneal (fibular) components are enclosed in a common sciatic nerve sheath, though their fibers remain separate throughout their length. The sciatic nerve’s tibial division supplies the adductor magnus (hamstring portion), biceps femoris long head, semimembranosus, semitendinosus, and posterior calf muscles (ankle and foot flexors); the peroneal division innervates the biceps femoris short head and the evertor and extensor muscles the lower leg.
MR imaging technique
Given the high soft tissue and contrast resolution provided by MR imaging, dedicated protocols optimized for peripheral nerve evaluation (magnetic resonance neurography [MRN]) are recommended for complete evaluation of sciatic nerve pathology. While protocols are institution-specific, they usually include high-resolution (>256 base resolution) and high contrast spin echo type 2-dimensional (2D) and 3-dimensional (3D) imaging sequences that allow multiplanar reformatting and facilitate lesion visualization and surgical planning with additional postprocessing. Homogeneous fat suppression is desirable to accurately detect and grade nerve signal abnormalities and is often achieved with a combination of short tau inversion recovery (STIR) and spectral adiabatic inversion recovery (SPAIR) sequences. , Diffusion-weighted imaging (DWI) is critical in the assessment of neurogenic tumors, aiding in the distinction between benign neoplasms with high apparent diffusion coefficient (ADC) values and malignancies with low ADC values. DWI is also a component of PSIF (reversed acronym of FISP, Fast Imaging with Steady State Precession), which is a 3D reversed gradient-echo steady-state sequence that provides high contrast between peripheral nerves and surrounding tissues, where the diffusion component helps suppress signal from small vessels, increasing nerve conspicuity. Quantification of nerve signal and tract abnormalities using fractional anisotropy (FA), and diffusion tensor imaging (DTI) is possible but reproducibility between different scanners needs to be better understood as newer techniques such as nonsingle-shot echo-planar DTI emerge. Quantitative efforts will likely be accelerated by the advent of reliable and accurate automated nerve segmentation aided by artificial intelligence.
Clinical signs, symptoms, and electrodiagnostic tests are critical to defining the anatomic region of interest and tailoring the protocol, although enlargement or extension of the field of view to include distal pathology may be necessary when the clinical picture is equivocal. Intravenous contrast is preferable if there is concern for neoplasm or infection, but of little added value in the assessment of compression neuropathy or secondary muscle denervation changes. Investigation of the sciatic nerve roots and proximal course of the nerve may benefit from oblique imaging, permitting long-axis and short-axis views as it exits the pelvis along the piriformis.
Given the variability in lexicon and subjectivity in grading nerve pathology on MRN, an MR-based guidelines and classification system, named Neuropathy Score Reporting and Data System (NS-RADS) was recently developed in an attempt to standardize terminology among radiologists and referring physicians, improve communication, and ultimately improve patient management. Briefly, in this system peripheral nerve pathology is classified into 1 of 7 domains: unremarkable (U), injury (I), neoplasia (N), entrapment (E), diffuse neuropathy (D), not otherwise specified (NOS), and postintervention state (PI), and then further stratified by severity. A subsequent multi-institutional validation study has shown promising results, with good accuracy and reliability to classify a variety of peripheral nerve pathologies among radiologists with different levels of experience.
Etiology of sciatic nerve abnormalities
In the following sections, we highlight representative examples of the types of pathology that can affect the sciatic nerve and its branches. Included are compression neuropathies at the sciatic notch, deep gluteal space, and fibular tunnel; both benign and malignant neoplasms; traumatic injuries; inflammatory and hereditary neuropathies; and post-treatment complications. Predisposing anatomic factors, the role of advanced MR imaging sequences, and the clinical treatment implications of these abnormalities are discussed.
Nerve Compression
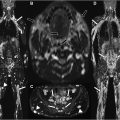
Beaton and Anson described 6 anatomic variants of the course of the sciatic nerve relative to the piriformis muscle. The classic type I (or A) anatomy where the entire nerve passes beneath the piriformis muscle fibers is observed in approximately 85% of cases, a frequency observed in a pooled analysis from over 6000 cadaveric dissections, and from the radiology literature. Almost all other cases of variant anatomy are type II (or B), where a slip of piriformis muscle divides the common peroneal nerve (CPN) and the tibial nerve ( Fig. 1 ). In one retrospective series of 755 examinations, type II anatomy was found in 13% of cases; only 2 other anatomic variants were seen in this large series (both type III). The importance of variant anatomy in the etiology of sciatic neuropathy, and specifically the role of an accessory muscle slip as a putative compressor in piriformis syndrome, remains controversial, with weak empirical evidence: in a retrospective study of 783 patients, piriformis syndrome was no more likely in variant hips (11%) than those with classic anatomy (9%, P =.39).

In another study, Russel evaluated 200 sacral plexuses in 100 patients undergoing routine MR imaging examinations and found that S2 and S3 nerve roots traverse the piriformis muscle in 75% and 97% of these cases, respectively. In addition to that, although piriformis muscle asymmetry has been appointed as a possible cause of piriformis muscle syndrome, muscle asymmetry was also found to be common, with up to 8 mm of asymmetry identified in this group of patients without sciatica symptoms.
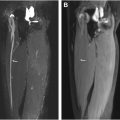
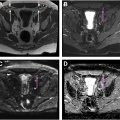
As piriformis anomalies alone fail to account for most patients’ symptoms, the concept of the deep gluteal space has evolved to encompass other potential sources of nerve entrapment and musculoskeletal dysfunction. Perhaps foremost among these are fibrovascular bands ( Fig. 2 ), but other entities include obturator internus/gemellus syndrome, quadratus femoris/ischiofemoral pathology ( Fig. 3 ), hamstring conditions, gluteal disorders and orthopedic causes. Fibrovascular bands are amenable to endoscopic release, with good functional outcomes, that may obviate the need for piriformis tenotomy. Occasionally perisciatic varicosities are observed ( Fig. 4 ), but their role in sciatica is controversial, with only case reports and small case series positing an association.



Clinics care points
- •
The classic type I (or A) sciatic nerve anatomy, where the nerve passes beneath the piriformis muscle, is seen in about 85% of cases, while type II (or B) anatomy, where a slip of the piriformis muscle divides the common peroneal and tibial nerves, occurs in approximately 13% of cases.
- •
The S2 and S3 nerve roots commonly traverse the piriformis muscle (75% and 97%, respectively), and piriformis muscle asymmetry is frequent, even in individuals without sciatica symptoms.
- •
The concept of the deep gluteal space has expanded beyond piriformis anomalies to include other causes of sciatic nerve compression, such as fibrovascular bands, obturator internus/gemellus syndrome, and quadratus femoris pathology, with surgical interventions showing promising outcomes.
Fibular Tunnel Entrapment
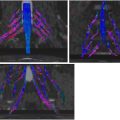
As the CPN (L4-S2) courses distally below the knee joint line, it has an oblique and superficial course making it susceptible to direct compression injury secondary to its anatomic position between the fibular head and the overlying subcutaneous tissues. An additional point of CPN injury is just distal to the fibular neck as it pierces the anterior muscular fascia of the leg to extend under the origin of the peroneus longus muscle. The most common site for compression is under a myoaponeurotic arch at the entrance of the fibular tunnel, comprised by muscle fibers and the aponeurosis of the soleus and peroneus longus muscles ( Fig. 5 ), typically exacerbated by forced inversion of the foot with stretching of the nerve and exacerbated by prolonged cross-legged or squatting positions. A pooled analysis of approximately 1000 cases revealed that the CPN normal cross-sectional diameter in the fibular tunnel is 8.4 mm 2 (95% confidence interval = 6.8–9.9 mm 2 , n = 1166), and at the popliteal fossa is 7.9 mm 2 (95% CI = 6.6–9.2 mm 2 ). A few uniform fascicles in both the deep and superficial peroneal nerve branches should be distinguishable at MR imaging. Findings of low-grade compression (NS-RADS E1) in the CPN are manifested as mild increases in nerve T2 signal intensity; more significant hyperintensity and morphologic flattening of the nerve denote E2 entrapment; severe T2 hyperintensity in the CPN with enlarged proximal and distal segments constitute E3 entrapment. While many cases resolve with conservative management, surgical release of the nerve improves strength and reduces pain in most patients.

Intraneural ganglion cyst is another increasingly recognized cause of compressive peripheral neuropathy, consisting of a mucinous lesion found within the epineurium of nerves, typically resulting in neurologic deficit due to displacement of the nerve fascicles. Ganglion cysts are joint derived, and in the lower extremities, most commonly affect the CPN, originating from the anterior portion of the superior tibiofibular joint. A ganglion cyst dissects proximally along the recurrent U-shaped articular branch ( Fig. 6 ), usually extending into the deep peroneal component of the CPN, producing the characteristic symptom of footdrop, from preferential compression of the deep peroneal fascicles. With continued increased intra-articular pressure, it may extend proximally into the peroneal division of the sciatic nerve, and in some cases, even reaching the buttock. , Treatment is usually surgical with complete resection not only of the intraneural cyst, but also of its articular branch which communicates with the joint. Simple cyst decompression or failure to excise the joint branch frequently results in recurrence.

Clinics care points
- •
The CPN is prone to compression at the fibular tunnel, with MR imaging severity grades from mild T2 signal changes (E1) to severe hyperintensity with nerve enlargement (E3).
- •
Intraneural ganglion cysts arise from the peroneal nerve’s articular branch supplying the proximal tibiofibular joint, often causing foot drop by compressing deep peroneal fascicles. Complete excision at the ganglion’s neck is needed to prevent recurrence.
Neoplasms
Neoplasms affecting the sciatic nerve can be intrinsic or extrinsic to the nerve fibers. Extrinsic tumors cause symptoms due to mass effect, and may displace the nerve or nerve roots without infiltrating nerve tissue, making nerve-sparing resection feasible ( Fig. 7 ). Intrinsic tumors may also be nonneurogenic, such as in benign nerve lipomatosis, classified as an adipocytic neoplasm by the World Health Organization (WHO) ( Fig. 8 ).


Among the intrinsic neurogenic tumors, the most common are benign peripheral nerve sheath tumors, with schwannomas being the most frequently encountered. Distinguishing between neurofibromas and schwannomas can be challenging, as both share overlapping imaging features: fusiform or round well-defined masses with a “tail sign” indicating neurogenic origin, and a “target sign” on fluid-sensitive and contrast-enhanced MR imaging. In neurofibromas, the target sign reflects a core of denser collagenous matrix and more hypercellular periphery; in schwannomas, the hyperintense myxoid Antoni B periphery surrounds the central more cellular Antoni A components ( Fig. 9 ). The presence of the target sign on ADC mapping is particularly predictive of benign peripheral nerve sheath tumor (PNST), as is a minimum ADC value of greater than 1 × 10 −3 mm 2 /s. Because they grow eccentrically, marginal excision of schwannomas is generally adequate and does not require sacrifice of the nerve or graft reconstruction.