The hip joint is home to a diverse range of neoplasms, as well as many pseudo lesions, including post-traumatic, infectious, and degenerative processes. Through careful evaluation of the clinical context, location, and imaging features, these entities can be distinguished, enabling accurate and efficient diagnosis. While not exhaustive, this article reviews a selection of benign, malignant, and non-neoplastic lesions affecting the hip bones, cartilage, and soft tissues, focusing on their notable imaging and pathologic features.
Key points
- •
Tumors of the hip represent a wide range of pathology including lesions of bone, cartilage, and soft tissues, many of which do not exclusively affect the hip and pelvis.
- •
Patient age and tumor location are key factors in forming the initial differential diagnosis; for instance, a lesion or fracture of the lesser trochanter in an adult should always raise suspicion of metastases.
- •
For bone lesions, radiographs (or computed tomography [CT]) are critical for determining the differential diagnosis.
- •
MR imaging plays a pivotal role in the diagnosis of hip tumors, many of which have distinguishing imaging characteristics.
- •
While cell origin remains a fundamental determinant of tumor type, genetic markers have become increasingly important in tumor identification, particularly in the context of modern therapeutic approaches.
Introduction
Hip tumors and pseudo-tumors encompass a broad spectrum of musculoskeletal pathology ranging from benign to malignant lesions originating from the femur and pelvic bones, as well as the adjacent soft tissues and hip joint. Accurate diagnosis, characterization, and staging of these lesions are crucial for effective clinical management, and MR imaging plays an indispensable role in this process. While many of the lesions addressed in this review are not exclusive to the hip and pelvis, this region presents unique challenges and adheres to important principles.
While the initial imaging obtained is typically a conventional radiograph, interpretation of hip lesions on radiographs can be challenging due to the superposition of normal osseous and soft tissue structures. The cortices of the iliac bones and sacrum are not well profiled on anteroposterior (AP) pelvic radiographs, and overlying bowel gas can further complicate the detection of osseous lesions. If a lesion is suspected, there should be a low threshold to proceed with computed tomography (CT) or MR imaging for further evaluation.
Due to the depth of pelvic structures, lesions arising from the pelvis typically present later and are often more advanced at diagnosis, while lesions from the proximal femur are more superficial and tend to present earlier. Generally, the hyaline cartilage of joints presents a barrier to tumor spread, and if transarticular spread is present, a more aggressive lesion should be suspected. Degenerative changes resulting in cartilage destruction can accelerate this transarticular spread.
Patient age and tumor location are particularly important in the hip region, as the frequency of benign and malignant hip tumors vary accordingly. In adults, pelvic tumors are most often malignant, while proximal femoral lesions are more frequently benign. Metastasis and myeloma are very common among adults over 40 years, and should be considered, especially when multiple lesions are present. In contrast, the majority of hip and pelvic tumors in pediatric patients are benign, such as simple bone cyst, osteoid osteoma, and fibrous dysplasia. When a malignant tumor is encountered, the child’s age is critical in forming the differential diagnosis, with considerations including neuroblastoma metastasis for those under 2 years, osteosarcoma and Ewing tumor for those between 5 years and older, and osteomyelitis and Langerhan Cell Histiocytosis.
Pelvic tumor mimics pose unique challenges, including those due to normal anatomy, trauma, post-procedural alterations, and infection. Normal synchondroses can mimic tumors in children and adolescents, especially when asymmetric closure is present. Additionally, variable amounts of hematopoietic bone marrow persist in the pelvis and proximal femora throughout life, leading to a potential diagnostic pitfall if mistaken for a tumor. Avulsion fractures in adolescents and insufficiency fractures in the elderly are common, and attention to lesion location is critical in making the correct diagnosis. Sites of prior biopsy and cancellous bone graft harvest should also not be mistaken for tumor. Their classic location and well-defined appearance, often with an overlying surgical scar, can aid in this important distinction. The infections most likely to mimic tumors are typically chronic such as tuberculosis, which can present with osseous destruction and soft tissue extension. Sarcoidosis is another classic mimicker, with multiple lytic, sclerotic, and mixed density lesions that can be mistaken for metastasis or myeloma.
In this article, the authors will review a selection of non-neoplastic, benign, and malignant lesions of the hip, with special attention to MR imaging findings that contribute to accurate diagnosis. While the lesions described in this article are not all-inclusive, the authors aim to highlight a selection of tumors and tumor-like lesions with classic or discriminating imaging features, offering useful insights to aid in their diagnosis.
MR imaging techniques
MR imaging plays a vital role in diagnosis and staging of hip lesions. It is important to note that the first imaging obtained should be a conventional radiograph, which is useful both as a low-cost screening tool and as a correlation to MR imaging. The imaging differential diagnosis is made with the radiograph or CT. Radiographs and CT are both superior to MR imaging in detection of lesion matrix and soft tissue calcifications. Magnetic resonance (MR) is outstanding for determining lesion extent. Bone scintigraphy is important in when localizing symptoms are absent and when assessing multifocal diseases, including metastatic disease and skip lesions. PET-CT and PET-MR are valuable in those lesions that are PET avid, such as lymphoma and selected metastases.
When performing an MR imaging of the pelvis or hip, the patient should be positioned supine with 15° internal rotation of the hips. A torso phased array coil is typically used; however, a body coil may be needed for larger patients. For optimized evaluation of smaller structures including the labrum or cartilage, surface coils are utilized, and intra-articular contrast may be administered. A 3 T magnet provides improved spatial resolution and improved signal-to-noise ratio over a 1.5 T magnet. Lower strength, 1.5 T magnets may best serve post-operative patients with metallic implants to reduce susceptibility artifacts.
The field of view (FOV) chosen for MR imaging depends on the lesion of interest and its extent. While minimizing the FOV optimizes spatial resolution and signal-to-noise ratio, it is imperative that all sequences include the full extent of the lesion. At least 1 sequence should also include a bony landmark for surgical planning, such as the anterior superior iliac spine or the greater trochanter. If the lesion is radiographically occult, a larger FOV should be obtained. As a general rule, skin markers should always be used in the region where the patient is symptomatic. In the initial pre-operative assessment of a lesion, the entire extent of the involved bone and, when necessary, the adjacent bone should be performed. This is valuable for assessing lesion extent and the presence or absence of skip lesions.
Essential sequences to evaluate a hip lesion include T1-weighted (T1W), T2-weighted (T2W), and short-tau inversion recovery (STIR) and/or fat-saturated T2-weighted (FS-T2W) imaging. Depending on the suspected lesion, additional sequences may be performed including post-contrast fat-saturated T1W (FS-T1W) and diffusion-weighted imaging (DWI). T1W imaging is particularly useful in evaluating anatomic detail and intraosseous tumor extent, including detection of skip lesions. STIR/FS-T2W sequences are important in detecting edema and fluid, making cystic lesions apparent and showing intraosseous and extraosseous tumor extent. However, it can be challenging to distinguish tumor from peritumoral edema. Additional sequences as well as in-phase/opposed-phase imaging and intravenous contrast enhancement may help in this differentiation. Homogeneity of the signal points toward edema over lesion, and when there is doubt, dynamic contrast enhanced MR imaging is useful in determining true tumor margins. STIR sequences are particularly sensitive for detecting soft tissue and marrow pathology, with greater homogeneity of fat saturation and reduced susceptibility artifacts compared to T2W sequences using frequency selective fat-saturation. Gradient echo (GRE) T2∗W sequences accentuate susceptibility effects, which is useful in detecting hemosiderin such as in tenosynovial giant cell tumor; however, these sequences are limited in patients with metallic hardware due to extensive artifact. GRE imaging can also be performed with 3 dimensional “volumetric” acquisition, which has several advantages including high-resolution and the ability to reconstruct images in any plane.
While intravenous contrast is not administered in routine hip MR imaging examinations, it is valuable in the initial assessment of bone and soft tissue lesions. Contrast enhancement can guide biopsy to a site of viable tumor, and in the post-treatment setting, enhancement along with diffusion restriction can enable distinction between necrosis and tumor recurrence. In the setting of infection, contrast enables differentiation of abscess and phlegmon, and improves detection of small sinus tracts. Intraarticular contrast is beneficial in evaluation of the labrum and cartilage, however, is usually unnecessary in tumor cases.
Protocols generally recommend obtaining at least 1 large FOV sequence to include the entire pelvis, followed by smaller FOV sequences of the individual hip of clinical concern. The large FOV sequence is typically a coronal T2 or STIR, extending from the superior sacrum/ilium to the pubic symphysis and including the anterior and posterior acetabular columns. Four planes of imaging are routinely performed: coronal, sagittal, axial, and axial-oblique. The axial plane is particularly useful in delineating a fat plane between the tumor and the neurovascular bundle of the hip. An axial oblique sequence is performed for labral evaluation, and therefore may be unnecessary for evaluation of a known tumor.
Dixon Versus Conventional Chemical-Shift Fat-Suppression
If available, DIXON sequences are superior to conventional chemical-shift fat-suppression sequences. T1 and T2-weighted DIXON sequences are helpful in the differentiation of underlying marrow pathology as opposed to yellow or hematopoietic marrow.
In-phase and Opposed-Phase Imaging
In-phase gradient echo is an excellent method to view the bone trabeculae for distortions that may be caused by neoplasms or for additional deposits of calcifications or ossifications within the marrow space causing distortions of the cancellous bone. Persistence of marrow signal on opposed-phase chemical shift imaging has been shown to reflect neoplastic marrow disease, when trying to differentiate vertebral body fractures from malignant marrow replacement.
Benign Synovial-Based Lesions
Tenosynovial giant cell tumor
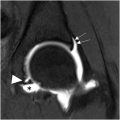
A mass arising within a joint that contains areas of low-signal intensity on MR on all sequences and demonstrates “blooming“ on gradient echo imaging (due to hemosiderin deposits) is the classic appearance of tenosynovial giant cell tumor ( Fig. 1 A–C ). Tenosynovial giant cell tumor may arise within a joint or within a tendon sheath. This lesion had previously been called “pigmented villonodular synovitis (PVNS),” when occurring within a joint, and giant cell tumor of tendon sheath, when occurring outside a joint. These lesions have been recognized as the same lesion, and are now called “tenosynovial giant cell tumor,” whether they arise inside or outside a joint. This lesion usually shows no calcifications on CT or MR and may present as a focal, nodular mass, the most common form. The lesion may present with inflammatory symptoms, and initially may be misdiagnosed as an infectious or inflammatory synovitis. ,

The other form is a diffuse, infiltrating type of lesion that cannot be eradicated by surgery, and even through the lesion is not a malignancy, this diffuse form has a very high recurrence rate, does not respect tissue boundaries, and is extremely locally invasive. Amputation has been required in some patients with this diffuse form, as the lesion will continue extending and can invade great vessels, the pelvis, abdomen, or chest. Recently, colony-stimulating factor 1 (CSF-1) overexpression has been identified in tenosynovial giant cell tumor. The CSF-1 receptor inhibitor pexidartinib has been approved for the treatment of tenosynovial giant cell tumor. This follows the trend of targeting the lesion mutations, rather than develop drugs aimed at specific tumors.
Synovial chondromatosis
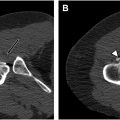
Nodules of hyaline cartilage arising within metaplastic synovium are synovial chondromatosis. The nodules may ossify, as they parasitize a blood supply from the synovium. A total of 5% to 10% of the nodules of synovial chondromatosis may have sufficient calcification or ossification, for those calcific bodies to be visible on plain films or CT ( Fig. 2 A, B ). Synovial chondromatosis may occur within joints or arise within tendon sheaths or other areas where synovium occurs ( Fig. 3 A, B ). On occasion, cases of synovial chondromatosis may mimic osteosarcoma, as the lesion may present as a solid block of calcified tissue. In other situations, synovial chondromatosis may not be identifiable on imaging, with only microscopic evidence of the cartilage nodules being present, but the patient may still be symptomatic. Cases of malignant degeneration of synovial chondromatosis to chondrosarcoma have been reported, but are extremely rare. ,


Benign Bone Tumors
Osteochondroma
Bone excrescences arising mostly from the metaphysis and extending toward the diaphysis and away from the joint with marrow communication and a cartilage cap describes most osteochondromas. Osteochondromas may be sessile or pedunculated, and when pedunculated, the stalk may fracture. The knee is the most common site for pseudoaneurysm formation, and when the overlying soft tissues are abraded and irritated by the osteochondroma, a bursa may form over the osteochondroma. Bursa formation is another common reason for a rapid increase in local pain along with soft tissue swelling and is not neoplastic. Malignant transformation of osteochondromas does occur, mostly to chondrosarcoma arising within the cartilage cap ( Fig. 4 A–C ), more commonly in the autosomal dominant Multiple Hereditary Osteochondromatosis and even more in the crossover condition that has features of both multiple osteochondromas and multiple enchondromas that has been named “Metachondromatosis.”

Intra-articular osteochondroma
Dysplasia epiphysealis hemimelica also known as “Trevor disease” has been identified with the moniker “Intraarticular Osteochondroma.” This entity is triggered by overgrowth of the growth plate and deformity of the articulation that is not purely an osteochondroma. , The imaging findings in this interesting condition are vital in making this diagnosis.
Enchondroma
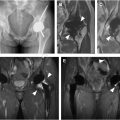
A small focus of benign hyaline cartilage within the marrow is an enchondroma. When many are present, the condition is known as “enchondromatosis (Ollier Disease),” with an increased risk of malignancy ( Fig. 5 A–D ). If also accompanied by soft tissue hemangiomas, the enchondromatosis condition is known as “Maffucci Syndrome.” Both Ollier Disease and Maffucci Syndrome have mutations of the IDH1 and IDH2 genes. The malignancy rate in Maffucci syndrome may exceed 40%.

Periosteal chondroma
An enchondroma that occurs on the surface of the bone is a periosteal chondroma. The imaging appearance is that of a lesion that bubbles off the cortex with a rim of periosteum. Matrix cartilaginous calcification is commonly found. Periosteal chondromas are frequent occurrences in patients with enchondromatosis (Ollier disease.)
Chondroblastoma
Chondroblastoma is a benign lesion of cartilaginous origin that is one of the classic end-of-bone lesions ( Fig. 6 A, B ). A histone 3.3 mutation, H3F3B has been identified as a marker for chondroblastoma, which is actually the same mutation that occurs in giant cell tumor of bone, just with a difference of the chromosome involved. Giant cell tumor of bone has the mutation on chromosome 1 and chondroblastoma has the mutation on chromosome 17. Periosteal reaction is often found in cases of chondroblastoma, and like many lesions that affect the end of bone, synovitis within the articulation may occur, without the tumor actually invading the joint. A chondroblastic variant of osteosarcoma has been described, which has a pathology appearance that may be confused with chondroblastoma.

Chondromyxoid fibroma
Chondromyxoid fibroma (CMF) is a rare tumor that has pathology features of cartilage, fibrous tissue, and myxomatous material. The lesion may occur within the marrow space or as a surface lesion. A chondromyxoid fibroma-like osteosarcoma has been described, which may present a pitfall to the diagnosis of CMF.
Osteoid osteoma/osteoblastoma
Osteoid osteoma and osteoblastoma have the same pathology findings and the same FOS/FOSB genetic mutations. The lesions are differentiated on the basis of size with 1.5 to 2.0 cm being used to differentiate the two ( Figs. 7–9 A–D ). Osteoid osteoma often presents with a history of pain relieved by analgesics. Reactive sclerotic bone often forms around a cortically-based osteoid osteoma, but not when the lesion occurs at the end of bone or within the marrow. This reactive cortical sclerosis is non-neoplastic and does not need to be treated. Osteoid osteomas are often round, but may be oval or even linear, making the diagnosis difficult. Due to their small size, osteoid osteomas may be confused for femoral neck herniation pits, or other benign lesions. After treatment by ablation therapy or surgical resection, recurrences are very difficult to localize, as the surround tissues are often distorted. The feared misdiagnosis is that of an intracortical or a high-grade osteosarcoma being treated as an osteoid osteoma. CT and Tc 99M Radionuclide bone scan are the optimal imaging tests for the diagnosis of osteoid osteoma. MR may demonstrate the lesion, especially following intravenous gadolinium administration, but some cases are notoriously difficult to locate on MR.



When the lesions are sufficiently large and the diagnosis of osteoblastoma is made, care needs to be given to assure that an osteoblastic or osteoblastoma-like osteosarcoma is not mistaken for an osteoblastoma. The entity of aggressive or epithelioid osteoblastoma has fallen out of favor, as the name is based upon the histologic features, but the lesion behavior and prognosis is the same as an ordinary osteoblastoma.
Intraarticular osteoid osteoma
When an osteoid osteoma occurs at the end of bone (see Fig. 8 ), the lesion does not have the characteristic surrounding sclerosis, and often presents with pain and focal osteopenia. CT often does not show the classic small circular lesion, and MR following intravenous gadolinium administration may be quite useful in making this diagnosis. As with other end of bone lesions, intraarticular synovitis may be present, confusing the diagnosis with infectious or inflammatory arthropathy being the primary considerations.
Malignant Bone Tumors
Chondrosarcoma
Malignant neoplasms of cartilage are chondrosarcomas, so long as they do not also contain tumor bone. There are many different types of chondrosarcomas and different grades, based upon the histology. Differentiation of low-grade lesions such as atypical cartilage tumors, some that were previously called grade 1 chondrosarcomas, from enchondromas may be quite challenging to the pathologist. In areas where the cartilage may have been subject to trauma, such as the extremities, the cellularity of the lesion may be increased, making the lesion appear more worrisome histologically. As a general rule, the larger the lesion and the more centrally located the lesion within the body, axial versus appendicular skeleton, the more worrisome it is for any given histology grade and size. ,
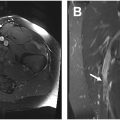
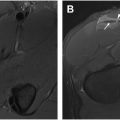
Clear cell chondrosarcoma
Clear cell chondrosarcoma is a subtype of chondrosarcoma that arises at the end of bone ( Fig. 10 A–C ). These lesions may mimic subchondral, degenerative cysts or may not even be detected on standard imaging, lost within the clutter of the classic degenerative changes that are often seen in older individuals. In a setting of femoral head osteonecrosis, a lesion of clear cell chondrosarcoma may be very difficult to identify. ,