When a suspicious finding is seen on a breast magnetic resonance imaging (MRI) examination and other imaging and clinical examinations are negative, the only way to perform a biopsy of the lesion is by using MRI guidance. All facilities that perform breast MRI should have the ability to perform MRI-guided biopsy. If it is not offered, patients must go elsewhere for the procedure, adding time, inconvenience, and cost to the process.
The American College of Radiology breast MRI accreditation program requires that facilities have MRI biopsy capability or have a formal relationship with another facility that will accept their patients for biopsy without repeating the examination. Not only is offering MRI-guided biopsy good medical practice that benefits patients more and more insurers are demanding accreditation of facilities in various modalities, including breast MRI, in order to receive reimbursement.
The steps in performing MRI-guided biopsy are similar to stereotactic breast biopsy, but there are factors and challenges that are unique to this procedure, including limited time of lesion visibility and lack of confirmation of lesion sampling. Despite this, experience with MRI-guided core biopsy has shown that this technique is effective with a high technical success rate. Lehman and colleagues reported successful tissue retrieval in 100% of 35 lesions. Liberman and coworkers were able to retrieve tissue in 95 (97%) of 98 biopsies, and Perlet and colleagues reported that 517 (96%) of 538 biopsies in their series could be completed.
The positive predictive value (PPV) of MRI-guided biopsies has been reported to be 18% to 61%, with most studies reporting a PPV of about 25% to 40%. The variation is likely caused by differences in patient populations and in interpretive criteria. It has been shown that the positive biopsy rate is higher for abnormalities found on diagnostic MRI than on screening studies and is highest for lesions found in the ipsilateral breast of women with a new diagnosis of breast cancer.
Patient and Lesion Selection
There are safety considerations in performing breast MRI, and some patients are not candidates for this examination. However, patients in whom MRI-guided biopsy is being considered have already had an MRI that identified the suspicious abnormality, making it less likely that safety is a contraindication. Aside from safety considerations, patient factors that are relative contraindications to MRI-guided biopsy are very few and are largely limited to bleeding diatheses.
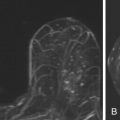
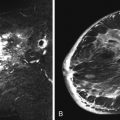
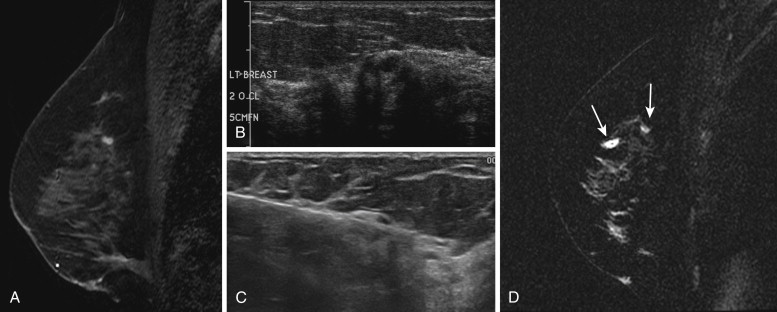
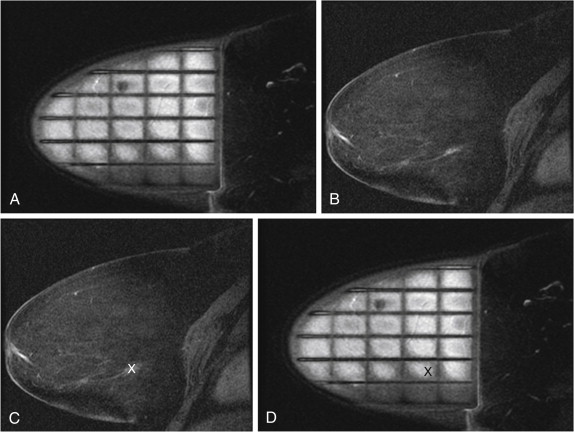
In terms of lesion selection, when a suspicious abnormality is seen on MRI and biopsy is recommended, the most recent mammogram should be reviewed to determine whether there is a corresponding abnormality that might guide excision. Targeted ultrasound should also be considered, especially for enhancing masses because biopsy under ultrasound guidance is faster, easier, and less expensive than MRI-guided procedures. Abe and coworkers reported finding a sonographic correlate in 57% (115/202) of suspicious MRI lesions for which targeted ultrasound was done, and Meissnitzer and colleagues reported finding a sonographic correlate in 56% (290/519). However, the Meissnitzer group also reported that on follow-up of 80 cases, the ultrasound finding was not the same as the MRI finding in 10 lesions (12.5%), 5 of which had been benign on ultrasound but actually proved to be malignant on MRI-guided biopsy. Therefore, whenever an ultrasound correlate is thought to be found for a suspicious MRI lesion and the biopsy results are benign and concordant, follow-up MRI should be done to be certain that the lesions are the same ( Figure 12.1 ).
When there is no sonographic or mammographic correlate and MRI-guided biopsy is necessary, it should be recognized that there are lesions that are not amenable to MRI-guided core biopsy. These include those that are very posterior in the breast, very superficial, close to the nipple, lesions in breasts that are very thin, and some lesions in women with implants. For suspicious lesions that are seen only on MRI for which tissue diagnosis is desired and for which MRI-guided core biopsy is not technically feasible because of lesion location or the size of the breast, MRI-guided wire localization with an MRI-compatible wire can be performed. These wires are virtually identical in design to conventional localizing wires that are placed under mammographic or ultrasound guidance, but they are thinner and tend to be more prone to transection in the operating room.
Patient Preparation Before the Procedure
Anticoagulation with warfarin (Coumadin) is usually reversed before performing core biopsy procedures. In some women, however, the medical risks of reversing anticoagulation may be so great as to require bridge therapy with low-molecular-weight heparin to safely prevent thrombotic or embolic events. Communication and cooperation between the radiologist who is recommending and/or performing the biopsy and the referring clinician are especially crucial in these patients.
Aspirin and nonsteroidal anti-inflammatory agents such as ibuprofen can also be associated with prolonged bleeding and are often stopped for 5 to 7 days before the biopsy. Melotti and Berg reviewed their experience with performing core biopsies on women on anticoagulation and reported that 3 (38%) of 8 patients on anticoagulation developed hematomas larger than 1 cm compared with 13 (8%) of 171 women who were not anticoagulated. However, none of the patients on either warfarin or aspirin who underwent stereotactic core biopsy with an 11-gauge needle required surgical intervention to stop bleeding or to evacuate a hematoma. They concluded that core biopsy in women on anticoagulation or aspirin therapy can be performed without stopping the medication if there are compelling reasons to do so, such as a patient’s inability to come back for the procedure at another time or possible noncompliance with a recommendation for biopsy if performed at a later date. Similar results were reported by Somerville and coworkers, who compared 200 women on anticoagulation with 855 women who were not and who underwent biopsy with 14-gauge or larger needles. There were significantly more bruises among women on anticoagulation but no significant difference in hematoma formation between the two groups, and none of the patients required surgical intervention. The numbers in these studies are small, however, with only 8 anticoagulated patients undergoing biopsy with an 11-gauge vacuum device in the report by Melotti and Berg and a total of 16 patients on warfarin in the report by Somerville and coworkers. Melotti and Berg report that they still reverse anticoagulation or withhold aspirin for 7 days in most cases, and Somerville and coworkers give the patients the option of stopping anticoagulation medications or proceeding with the biopsy. If a patient on warfarin elects not to stop anticoagulation, they recommend that the international normalized ratio be checked to be certain the patient is not above the therapeutic range.
With regard to prophylactic antibiotics, there is no evidence that these are indicated prior to MRI-guided core biopsy.
Informed Consent
As with any interventional procedure, informed consent should be obtained after the risks and benefits are explained to the patient. There are little data on the incidence of complications associated with MRI-guided biopsies. There is information, however, on other core biopsy procedures, namely those done with stereotactic and ultrasound guidance, showing a low incidence of complications. Serious complications from vacuum-assisted core biopsies are infrequent, reported in about 1% of cases. Infection after core biopsy is another uncommon occurrence.
Besides potential complications, a discussion of the need for clip placement, as well as the need for follow-up MRI if the results of the biopsy prove to be benign, is sometimes included. Additionally, it is often a good idea to formulate a plan before the biopsy for conveying results to the patient, including when pathology results can be expected, who will convey them, and how this will be done (either in person or by telephone).
Equipment Selection
Magnet Strength
Equipment is available for performing MRI-guided core biopsies on both 1.5 and 3.0 Tesla (T) magnets. There is no evidence that doing the biopsy on a machine with a different field strength from that in which the diagnostic study was done poses any problems. For example, a biopsy can be done on a 1.5 T scanner after a diagnostic study is done on a 3.0 T magnet, and vice versa.
Targeting Equipment
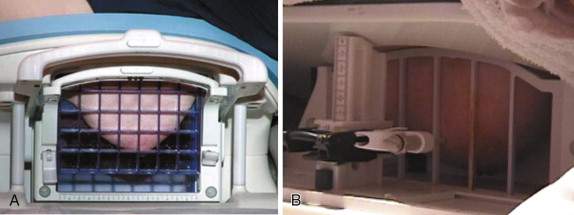
A variety of different devices that add onto the breast coil are available that allow for targeting of the enhancing lesion. These devices use either a grid compression plate or a pillar-and-post apparatus ( Figure 12.2 ). All allow approach from the lateral aspect of the breast, and many also allow medial access. Newer coils are being developed that will enable approach from other directions.
Vacuum-Assisted Devices
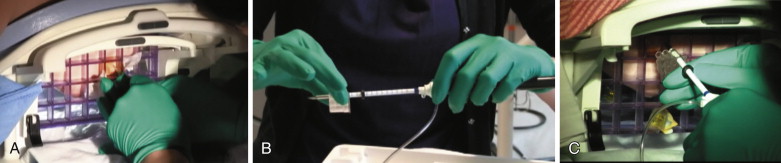
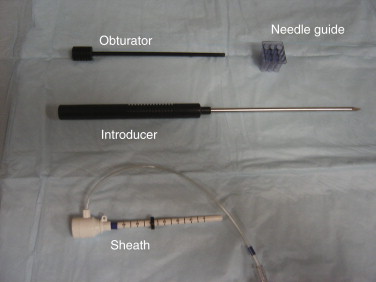
A number of different devices are used for vacuum-assisted MRI core biopsies ranging from 7- to 11-gauge needles. Vacuum-assisted core biopsy with needles that are 11 gauge or larger have been shown to result in more accurate results than 14 gauge automated core biopsies. All have basically the same design, with a plastic sheath that allows the calculated depth of the lesion to be set. A sharp introducer is placed through the sheath and allows the sheath to be positioned in the breast. There is a plastic obturator that is then placed to allow scanning to confirm accurate positioning of the sheath. Finally, the sampling device is placed through the sheath, and tissue samples are obtained. An example of an introducer set is shown in Figure 12.3 . Some of the vacuum-assisted devices have a self-contained vacuum, but most have a console that provides the suction and remains outside of the magnet room, connected to the needle by long tubing. With some of the vacuum-assisted devices, the cores are collected automatically in a chamber, and with others the cores are retrieved manually with each pass. Following the procedure, a variety of MRI-compatible clips are available that can be placed at the biopsy site.
Biopsy Technique
Before an MRI-guided biopsy is performed, the diagnostic study that prompted the biopsy recommendation should be thoroughly reviewed and the approach planned. This is particularly important for MRI-guided procedures because of limited time of lesion visibility owing to washout of contrast and enhancement of the surrounding normal parenchyma over time. The reported average time to perform a vacuum-assisted core biopsy of a single lesion under MRI ranges from 35 to 58 minutes. Scheduling one or one-and-a-half hours of magnet time is usually sufficient. Multiple and bilateral lesions will, of course, require more time. Lehman and coworkers reported that the mean time for biopsy of multiple lesions in one breast was 59 minutes and for bilateral lesions was 64 minutes, compared with a mean of 38 minutes for a single lesion. Similarly, Liberman and colleagues reported a mean time of 35 minutes for a single lesion and 65 minutes for two lesions.
Biopsy coils generally allow approach from either the lateral or medial surface, and the approach that represents the shortest distance to the lesion should be chosen. In general, a sagittal sequence is used to determine which opening in the grid is to be used. It is useful for facilities that acquire primary diagnostic images axially either to obtain a sagittal sequence as the last postcontrast series or to acquire images with isotropic voxels that allow subsequent reformatting in any plane without substantial loss of resolution. Some biopsy coils allow a fenestrated grid to be placed laterally and medially at the same time. This is advantageous when the lesion is close to the midline and allows more flexibility of approach. Care should be taken, however, not to confuse the medial and lateral aspects of the breast on the subsequent images. One way of ensuring easy differentiation is to place one fiducial on one side and two on the other, or to place the fiducial marker on the superior aspect of the breast on one surface and inferiorly on the other.
Multiple Lesions
Multiple lesions in one breast can often be biopsied during the same session. Coils that allow both a lateral and medial approach at the same time can be used if the lesions are in opposite sides of the breast. Bilateral lesions can also be successfully biopsied during the same session if the lesions are accessible from the lateral approach in each breast. If not, they will likely need to be sampled on different days.
Patient Positioning
As with diagnostic MRI, the intravenous line should be placed before the patient is put in the magnet and the patient should be positioned in the breast coil by either an experienced technologist or by the physician who is doing the procedure. Often, facilities that are just starting to perform MRI-guided biopsies will have mammography technologists who are experienced in positioning for stereotactic biopsies position the patient’s breast in the coil, because the techniques are similar. The breast tissue should be pulled into the coil to maximize the amount of tissue visualized, and then compression can be applied. The compression should be firm enough to ensure that the lesion does not move, but not so much as to compromise enhancement. Time and care should be taken to maximize patient comfort to minimize patient movement during the procedure.
Contrast Administration
Most abnormalities that are undergoing MRI-guided biopsy are not seen without the administration of intravenous contrast. Power injection is not necessary because kinetics will not be assessed. However, giving a contrast agent without having to move the patient out of the magnet—either with a power injector that is connected to the IV line beforehand or hand injecting through connecting tubing—is desirable to minimize the possibility of patient motion.
Targeting
All of the commercially available computer-aided detection (CAD) products have biopsy-targeting software that can be used to target lesions. These programs usually require use of a particular fiducial marker and can automatically calculate lesion location in relation to the position of the fiducial marker from either an axial or a sagittal sequence ( Figure 12.4 ). However, biopsies can be quickly and successfully performed without these programs, and computer malfunction or software problems can occur. Therefore knowledge of the procedure of manual lesion targeting without computerized software is essential. Targeting is slightly different between a grid compression device and the pillar-and-post method. There are advantages and disadvantages to each. Targeting with the grid device is often simpler and faster. The pillar-and-post technique allows angulation of the needle to access lesions close to the chest wall, and the needle entrance site in the skin is more readily visualized.
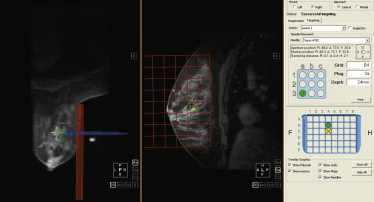
Targeting with a Grid Device
To locate an enhancing lesion in the breast, a fiducial marker needs to be placed and included on the images. Several options exist for fiducial markers, including simple vitamin E capsules. Some of the biopsy grids have built-in markers. If a marker is placed manually, it can be put either in the grid opening that is likely to be directly over the lesion as determined by the diagnostic study or in a grid opening that is not likely to be over the lesion, so that the fiducial does not have to be moved prior to placing the needle.
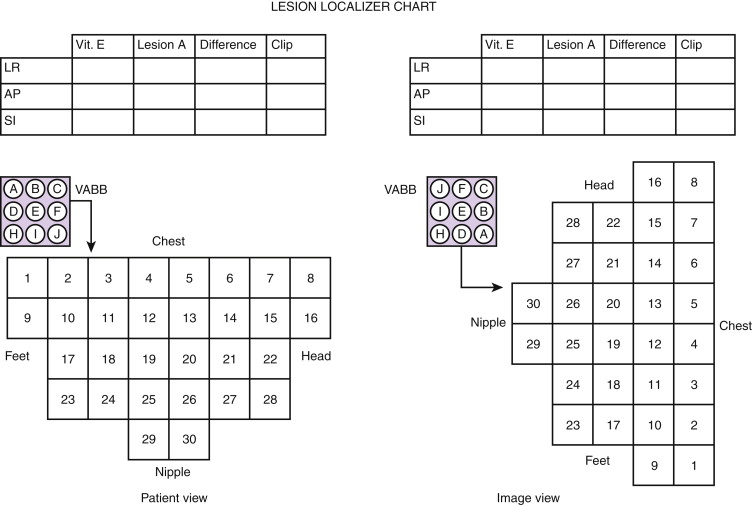
For the manual grid technique, a sagittal sequence is needed, either acquired directly or reformatted with high enough resolution to visualize the lesion. A precontrast T1-weighted sequence is usually obtained to ensure that technical factors such as positioning and fat suppression are adequate and that the fiducial is seen. Additionally, in occasional cases subtraction images help clinicians visualize the enhancing lesion, and having a precontrast sequence allows for subtraction to be performed. Once the lesion is identified, the appropriate grid opening and appropriate hole in the needle guide are identified by determining the relative position of the lesion to the fiducial marker. This determines the x and y coordinates of the lesion. The lesion depth, the z coordinate, is easily determined by counting the number of slices between the skin surface and the center of the lesion. The number of slices is then multiplied by the slice thickness to obtain the depth ( Figure 12.5 ).
Because the orientation of the image on the imaging monitor is different from the orientation of the patient on the scan table, it is relatively easy to choose the incorrect grid opening. To avoid this mistake, diagrams of the grid can be used to mark the location of the fiducial and the lesion ( Figure 12.6 ). The diagram can then be brought into the magnet room and turned to orient it in the same position as the patient.
Targeting with a Pillar-and-Post Device
Manual targeting when using a pillar-and-post device can be done with either an axial or a sagittal sequence. Once the patient is positioned, compression is applied and the pillar is set to zero for both the x and y axes. Again, a fiducial marker is placed and included in the precontrast scan. Contrast is administered, and an image sequence, usually in the axial plane, is obtained. The position of the fiducial is marked on the slice on which it is seen, and a reference line is placed on the workstation across the breast. The number of slices between the fiducial and the lesion is counted and multiplied by the slice thickness. This gives the location in the x (horizontal) axis. The distance from the lesion to the fiducial reference line determines the y axis (vertical) location. The pillar is then moved to these coordinates, the fiducial moved, and the patient rescanned to be certain that the location is correct. The depth of the lesion is then measured from the skin surface to determine the z position.
Local Anesthesia
Premedication with an oral anxiolytic agent is not usually necessary but can be helpful for particularly anxious patients. Local anesthesia, typically 1% Lidocaine without epinephrine, is administered with a 25-gauge needle to raise a skin wheal. Buffering the lidocaine with sodium bicarbonate will neutralize the acidic pH to decrease the initial stinging sensation. Depending on lesion depth, deeper anesthesia can be administered before inserting the biopsy needle. Some biopsy devices have the capability of delivering local anesthetic simultaneously with tissue acquisition. Lidocaine (1%) with epinephrine is often used for this deeper anesthesia unless there is a relative contraindication to the use of epinephrine, such as heart disease or arrhythmia.
Tissue Acquisition
Once the lesion is targeted, the appropriate grid and needle- guide opening determined, and local anesthesia delivered, a skin nick can be made with a scalpel to facilitate needle insertion. The introducer used with biopsy kits is so sharp that it can be placed in the breast without a skin nick, but this results in a circular wound rather than the linear one created by a scalpel and can result in a more visible scar.
When a nick in made with a scalpel and the grid device used, the tip of the introducer should be placed in the nick before the needle guide is popped into the grid to ensure that the needle does not make a separate wound ( Figure 12.7 ). The introducer is then placed to the predetermined depth and replaced by the nonmetallic obturator to allow scanning to determine accuracy of positioning.