Computed tomography enterography, magnetic resonance enterography, and bowel ultrasound are complementary tools central to diagnosing and monitoring Crohn disease. These modalities can identify active inflammation, penetrating, and stricturing disease. Crohn disease must be monitored frequently to guide therapy, and resolution of inflammation on imaging correlates directly with steroid-free clinical remission. While disease activity assessment is qualitative, newer quantitative techniques to assess active inflammation are emerging. These as well as other techniques, such as contrast-enhanced ultrasound (US) and US elastography, will offer new tools for future radiologists.
Key points
- •
Computed tomography enterography (CTE), magnetic resonance enterography (MRE), and bowel ultrasound (BUS) are complementary modalities in diagnosis and monitoring of active inflammation, penetrating, and stricturing Crohn disease.
- •
Optimal oral contrast intake, timing, sequences, and special scenarios are highlighted for acquiring robust CTE and MRE.
- •
BUS is gaining traction as an option to diagnose and follow patients with inflammatory bowel disease (IBD) with grayscale, color Doppler, contrast-enhanced techniques, quantitative assessment, and elastography.
- •
Disease must be monitored frequently to guide therapy as resolution of inflammation on imaging correlates directly with steroid-free clinical remission.
- •
Newer techniques to quantitatively assess inflammation and abbreviated protocols will be future tools in guiding Crohn disease management.
Introduction
Crohn disease (CD) can involve any portion of the gastrointestinal tract and commonly involves the small bowel. As most small bowel is largely inaccessible by standard endoscopic techniques with falsely negative endoscopy in over 50% of patients, imaging is critical to assessment of small bowel disease activity. Computed tomography enterography (CTE) and magnetic resonance enterography (MRE) are recommended for evaluation of small bowel CD by the Society of Abdominal Radiology (SAR), American Gastroenterological Association, and the Society of Pediatric Radiology. This article reviews CTE, MRE, and bowel ultrasound (BUS) imaging techniques, small bowel CD imaging findings, CD monitoring and treatment response, and new developments.
Imaging technique
Computed Tomography Enterography
CTE requires administration of intravenous and neutral oral contrast (less than 20–30 HU). Positive oral contrast agents (eg, gastrografin and barium) obscure bowel wall, precluding visualization of mural enhancement, which is critical to assessment of CD activity. Neutral oral contrast agents, such as a commercial flavored sugar beverage (Breeza; Beekley Medical), polyethylene glycol, mannitol, milk, water, or lactulose, distend bowel and allow assessment of mural enhancement. In a survey of 16 institutions by the SAR Disease Focused Panel on Inflammatory Bowel Disease (SAR IBD DFP), Breeza was most commonly utilized.
Since uniform small bowel distension is important, 1000 to 1350 mL of oral contrast (20 mL/kg in children) should be continuously ingested over 30 to 60 minutes with nurse or technologist supervision, and imaging should be performed 45 to 70 minutes after initiating drinking. In many busy practices, imaging is performed within 60 minutes to maintain workflow. For patients with prior small bowel resections, imaging should be performed within 30 to 45 minutes. A cup of water is administered on the table prior to the scan to distend the stomach, duodenum, and proximal jejunum. If water is used as the enteric contrast agent, imaging should be performed earlier after drinking (eg, 30 minutes) as water is readily absorbed and may result in poor small bowel distension. , ,
Intravenous contrast is required for CTE with imaging in either enteric phase (40–50 seconds post contrast-injection) or portal venous phase. Vandenbroucke and colleagues demonstrated no difference between 40-second and 70-second (portal venous) contrast timing in evaluating the presence or absence of CD activity. In a survey of institutions represented by the SAR IBD DFP, there was variability in contrast timing technique with 10 institutions imaging during enteric/late arterial phase, 6 imaging during portal venous phase, and 13 imaging with fixed timed delay of greater than 70 seconds. Twenty-five percent of surveyed institutions routinely create coronal maximal intensity projection images to facilitate evaluation of mesenteric vein patency.
Benefits of CTE include fast acquisition time, spatial resolution, and minimization of motion artifacts when compared to MR imaging. Patients with CD often require repeat imaging and despite radiation exposure reduction techniques, ionizing radiation remains an important consideration.
Magnetic Resonance Enterography
MRE is valuable in the evaluation of disease activity. A key advantage of MRE includes lack of ionizing radiation, especially since an analysis suggested 15.5% of CD patients had cumulative effective dose greater than 75 mSv. MRE also offers better contrast resolution, making bowel edema easily visible. Additionally, in MRE, sequences are obtained at multiple time points, increasing the likelihood each bowel segment is distended on at least some sequences, allowing radiologists to delineate transient underdistension or peristalsis from pathologic luminal narrowing. Key MRE sequences are detailed in Table 1 .
| Sequence | Advantages |
|---|---|
| Fluid sensitive (T2-weighted imaging (T2WI), Single shot fast spin echo (SSFSE), balanced steady-state free precession (b-SSFP), fat-saturated T2WI | Detection of mural edema Detection of intramural fat, a sign of longstanding inflammation Detection of perienteric edema , |
| Post-contrast T1- weighted imaging (T1WI) with multiple post-contrast phases | Detection of inflammation, vascular complications, penetrating disease , Dynamic enhancement characteristics may differentiate inflammation from fibrosis |
| Diffusion-weighted imaging (DWI) |
|
To maximize image quality, optimal patient preparation is essential. Patients should fast 4 to 6 hours prior to reduce intraluminal filling defects and improve oral contrast consumption. As with CTE, MRE requires oral contrast not only for adequate bowel distension, but also to displace intraluminal air that would cause susceptibility artifact. Jesuratnam-Nielsen et al. found magnetic resonance (MR) without oral or intravenous contrast may overestimate segments of bowel wall thickening and reduce sensitivity of diffusion-weighted imaging (DWI) in detecting inflammation. Preferred agents are hypointense on T1-weighted imaging (T1WI) and hyperintense on T2-weighted imaging (T2WI) to better evaluate bowel wall. While protocols may vary, 1 to 1.5 L is often administered with 250 to 500 mL immediately prior to scanning to increase gastric and duodenal distension. Patient positioning is variable. Some radiologists prefer the prone position because the mild pressure on the anterior abdominal wall can promote separation of small-bowel loops; however, 75% of surveyed centers perform supine imaging.
To reduce bowel motion, paralytic agents (most commonly Glucagon in the United States) are administered. Most centers administer agents immediately before diffusion-weighted and T1-weighted post-contrast imaging to maximize anti-peristaltic effects for these particularly motion-sensitive sequences. ,
MRE has become an increasingly popular option in CD with many studies demonstrating equivalent performance to CTE in disease detection in both adults and children. While CTE and MRE can equally detect active small bowel inflammation, CTE better identifies perienteric features of inflammation (engorged vasa recta, edema, fibrofatty proliferation, lymphadenopathy) as evaluating mesentery is challenging by MRE due to motion sensitivity; however, MRE may better identify fibrosis with greater sensitivity and accuracy (perhaps due to its better contrast resolution and temporal imaging protocol).
Bowel Ultrasound
BUS is often well tolerated by patients and less expensive than MRE. Patients are asked to consume no food by mouth for 4 hours before the examination and are encouraged to drink non-carbonated beverages prior to scan to reduce bowel gas and increase bladder distension. Unlike CTE and MRE, dedicated oral preparation is avoided to maximize compliance and patient comfort. BUS also allows for dynamic maneuvers such as compression. As with all ultrasound (US), BUS is operator dependent and limited in patients with larger body habitus or excessive bowel gas.
Low-frequency probes (5–9 MHz) provide a broad overview of intestinal anatomy while high-frequency probes (12–15 MHz) provide high-resolution pictures with excellent visualization of bowel wall layers. Harmonics can also be used to help delineate these bowel layers. A step-by-step approach is detailed in Table 2 . Pathologic segments should be documented in their entirety in longitudinal and transverse dimensions. Cines should originate at normal margins of pathologic segments and include the entire segment.
| Transducer Used or Doppler | Assessment |
|---|---|
| High frequency |
|
| Low frequency |
|
| High frequency |
|
| Doppler |
|
Imaging findings
Small Bowel Inflammation
Computed tomography enterography and magnetic resonance enterography
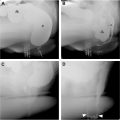
MRE findings of active inflammation include bowel wall thickening, increased T2 signal, mural hyperenhancement, and diffusion restriction. Discontiguous involvement or “skip lesions” are also a specific feature of CD. CTE assessment for inflammation is made primarily based on mural thickening and hyperenhancement ( Fig. 1 A–C ).

The most specific enhancement pattern for active disease is asymmetric mural hyperenhancement ( Fig. 2 ) preferentially involving the mesenteric border. Associated with this asymmetric enhancement along the mesenteric border is sacculation along the anti-mesenteric border. Another commonly identified pattern is stratified mural hyperenhancement— either bilaminar (inner wall layer hyperenhancement) or trilaminar (inner and outer wall layer hyperenhancement). While nonspecific for CD-related inflammation, mural hyperenhancement can also be homogeneous and symmetric.

Normal small bowel wall is typically T2 hypointense and thinner than 2 mm. Thickness greater than 3 mm or T2 hyperintensity may indicate edematous, actively inflamed small bowel. T2 fat-saturated sequences are particularly helpful for assessing edema and for differentiating mural edema from fat. High T2 signal within bowel wall is specific for severe active inflammation ( Fig. 3 A–E ).

On diffusion-weighted sequences, diffusion restriction alone is a nonspecific sign of mural inflammation and can be exaggerated by underdistention. , However, when used in conjunction with other findings of small bowel inflammation, it correlates well with endoscopy.
Ulcerations are breaks in the intraluminal surface that do not extend beyond the bowel wall and are a sign of severe active inflammation (see Fig. 1 A). Ulcerations on imaging correlate with severe disease on endoscopy. Diffusion-weighted imaging, particularly apparent diffusion coefficient (ADC) values, have been shown to be helpful in detecting ulcerations.
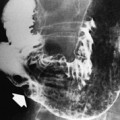
There are several mesenteric findings that are classically associated with active inflammation including but not limited to perienteric edema, engorged vasa recta, fibrofatty proliferation, and mesenteric vein thrombosis . Each of these imaging features can be depicted on CTE, MRE, and US (see Fig. 3 ; Figs. 4–7 ). Although not specific for CD or ulcerative colitis, these findings are ancillary to inflammatory bowel disease (IBD) diagnosis. Mesenteric venous thrombosis (MVT) is associated with a higher rate of bowel stenosis and IBD-related surgery. Acute MVT on computed tomography (CT) is a central low-attenuation filling defect surrounded by a well-defined rim-enhancing venous wall. Chronic MVT is associated with decreased caliber of the vein and complete occlusion. Acute MVT progesses toward chronic MVT and collaterals develop. Recognition of acute MVT is important, as early anticoagulation may lead to an improved rate of recanalization.




Finally, the ability to evaluate for extra-enteric findings, such as primary sclerosisng cholangitis, sacroilitis, avascular necrosis, urolithiasis, cholelithiasis, pancreatitis, and perianal disease, offer a distinct advantage of MRE and CTE.
Ultrasound
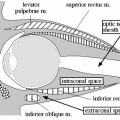
On BUS, the mucosa-luminal interface (hyperechoic), deep mucosa (hypoechoic), submucosa (hyperechoic), muscularis propria (hypoechoic), and serosa (hyperechoic) are visible ( Fig. 8 A, B ). Adjacent mesentery should be echogenic without thickening and normal bowel should demonstrate compressibility and peristalsis and little vascularity. BUS findings include mural thickening, loss of normal bowel wall stratification, loss of compressibility, loss of peristalsis, ulcers, and hyperemia of the bowel wall and often of the associated mesenteric fat. Anecdotally, when assessing hyperemia of the bowel wall, it is important to place the color Doppler over the region of the bowel wall separate from the mesentery with the correct pulse rate frequency so as to capture true vascularity in the bowel wall ( Fig. 9 A–C ). This will help distinguish between hyperemia in the bowel wall versus mesenteric hyperemia.


Bowel wall thickening is the most important finding in assessing the presence of active inflammation. It has the greatest inter-reader agreement and is most closely correlated with clinical activity markers, such as the Harvey Bradshaw index and Crohn Disease Activity Index. Recent expert consensus recommends a 3-mm threshold for wall thickening in small and large bowel, which has 88% sensitivity and 93% specificity for active inflammation. , Bowel wall thickness and mesenteric inflammation may correlate with the need for surgery. Per the METRIC trial, there is substantial inter-reader agreement for disease presence (kappa 0.64 for new diagnosis and 0.63 for relapsing cohort), and there was slight-fair agreement on disease extent (kappa of 0.27 for new diagnosis and 0.11 for relapsing cohort).
Strictures
While both fibrosis and smooth muscle hypertrophy contribute to stricture formation, smooth muscle hypertrophy predominates in the small bowel. , Most strictures contain both active inflammation and fibrosis. Strictures with predominantly active inflammation may respond to medical therapy, while primarily fibrostenotic strictures are more likely to require surgical or endoscopic intervention. While this determination can be difficult, particularly as inflammation and fibrosis often coexist, signs of fibrosis include upstream dilation greater than 3 cm, small bowel feces sign, and enhancement gain on the delayed phase compared to portal venous and arterial phases. , Radiologically, strictures are defined as fixed luminal narrowing (50% less than adjacent normal bowel) with wall thickening and upstream bowel dilation greater than 3 cm ( Figs. 10 and 11 ). The SAR IBD DFP consensus criteria also describe a “probable stricture” as bowel with wall thickening and persistent luminal narrowing on multiple MRE sequences or multiple examinations with less than 3 cm upstream dilation. Current consensus guidelines maintain that luminal narrowing, wall thickening, and upstream dilation are more specific because active inflammation can cause both luminal narrowing and wall thickening. , It is also important to distinguish between naive strictures and anastomotic strictures, as these can have different etiologies and treatment responses.