Pediatric patients are affected by a wide variety of pulmonary vascular diseases ranging from congenital anomalies diagnosed at birth to acquired diseases that present later in childhood and into adolescence. While some pulmonary vascular diseases present similarly to those seen in adults, other forms are unique to children. Knowledge of the characteristic imaging features of these diseases is essential to facilitate prompt diagnosis and guide clinical management.
Key points
- •
Children can be affected by a wide range of pulmonary vascular diseases, some of which present in infancy and others of which present later in childhood or adolescence.
- •
Some diseases present differently in children than adults due to disease heterogeneity, while others may present similarly in children and adults.
- •
Contrast enhanced computed tomography and magnetic resonance angiography provide detailed depiction of pediatric pulmonary vascular diseases.
Introduction
A wide spectrum of pulmonary vascular disorders causes considerable morbidity and mortality in the pediatric population. These disorders may present soon after birth or later in childhood or adolescence. Some of these disorders manifest similarly as in adults, while others are unique to neonates or children or have a much stronger association with genetic variants compared to adults. Anatomically, these disorders may primarily affect either the large pulmonary arteries or veins and be amenable to confident diagnosis by chest computed tomography (CT) or MR imaging angiography or involve the microvasculature at the level of the arterioles, venules, or capillaries with the imaging findings dependent on the secondary effects such as pulmonary hypertension, edema, or hemorrhage. Manifestations of these disorders can be restricted to the cardiopulmonary structures or associated with systemic diseases, syndromes, or anomalies in other body regions that serve as diagnostic clues. Although definitive diagnosis of some of these disorders depends on genetic testing or lung biopsy, appropriate imaging technique and knowledge of the characteristic imaging features of these disorders are essential to facilitate prompt diagnosis and guide clinical management.
Large pulmonary vessel developmental anomalies
Total Anomalous Pulmonary Venous Return/Partial Anomalous Pulmonary Venous Return and Scimitar Syndrome
Anomalous pulmonary venous return encompasses a spectrum of abnormal pulmonary venous connections in which at least 1 pulmonary vein drains outside of the left atrium. This results in an extracardiac left-to-right shunt. Total anomalous pulmonary venous return (TAPVR) requires a right-to-left shunt for survival via an atrial or ventricular septal defect, or patent ductus arteriosus. The 4 types of TAPVR classified based on the level of drainage include supracardiac, cardiac, infracardiac, and mixed types. Anomalous drainage via a vertical vein into the left brachiocephalic vein is most commonly encountered. , Poorer prognosis after TAPVR repair is highest in neonatal patients, patients with preoperative pulmonary venous obstruction, and in infracardiac and mixed types. , Computed tomography angiography (CTA) plays an important role in the preoperative morphologic evaluation of TAPVR ( Fig. 1 A, B ).

Patients with partial anomalous pulmonary venous return (PAPVR) do not typically exhibit symptoms in infancy and childhood, , and PAPVR is further discussed in Phan and colleagues’ article, “ Congenital Pulmonary Vascular Anomalies and Disease ,” in this issue. Scimitar syndrome is a rare congenital anomaly with a constellation of abnormalities including PAPVR and varying degrees of hypoplasia of the right lung and right pulmonary artery. Infantile presentation is most severe, with patients more likely to have aortopulmonary collaterals, congenital heart defects, pulmonary hypertension, non-cardiac anomalies, and higher rates of mortality than those presenting in childhood. Childhood presentation typically manifests with recurrent pneumonia, murmur, and less significant associated defects. Typical radiographic findings include hypoplastic right lung and dextroposition of the heart. The anomalous vein may be visible on chest radiography but is better delineated on CTA ( Fig. 2 A, B ).

Pulmonary Sling
Pulmonary sling is a congenital vascular anomaly with an aberrant left pulmonary artery arising from the right pulmonary artery that courses between the trachea and esophagus to the left lung. Type 1 pulmonary slings are less common and usually associated with tracheobronchomalacia, but normal bronchial branching. , Type 2 slings are more common and associated with malformations of the tracheobronchial tree, particularly tracheal stenosis due to complete cartilaginous rings. , As a result of associated tracheobronchial abnormalities, most patients demonstrate signs and symptoms in the first year of life, including wheezing, dyspnea, and stridor. , While chest radiographs may give clues to tracheobronchial abnormalities, including a low T-shaped carina and asymmetric lung hyperinflation, , , CTA is preferred for clear delineation of the pulmonary arteries and tracheobronchial anatomy ( Fig. 3 A, B ).

Pulmonary Atresia
Pulmonary atresia is a congenital anomaly with lack of continuity between the right ventricle (RV) and pulmonary arteries with or without a ventricular septal defect. Pulmonary atresia with ventricular septal defect represents the most severe form of Tetralogy of Fallot. Pulmonary arterial supply is from systemic arterial circulation via a patent ductus arteriosus (PDA) and/or major aortopulmonary collateral arteries (MAPCAs), typically from the descending aorta. While MAPCAs and a PDA can co-exist, they will not supply the same lung. Those with ductal supply of the pulmonary arterial system typically present with normal distribution of the intrapulmonary arterial tree, whereas those with MAPCAs frequently demonstrate arborization abnormalities. CTA accurately characterizes the branch pulmonary artery anatomy and pulmonary arterial blood supply necessary for surgical planning. Pulmonary atresia with intact interventricular septum presents with varying degrees of RV hypoplasia and hypertrophy, and frequent coronary anomalies. Patients presenting with a thin and dilated RV and massively dilated right atrium demonstrate significant tricuspid regurgitation. , Those with significant hypertrophy and hypoplasia of the RV have hypoplastic tricuspid valves and egress of blood from the RV through ventriculocoronary connections (sinusoids) thought to occur due to elevated RV pressure during development. Notably, because blood flow to the pulmonary arteries is dependent on a PDA, MAPCAs are rarely present. CTA can be used to delineate sinusoids, assess for coronary arterial stenosis or atresia, and define pulmonary arterial anatomy.
Proximal Interruption of the Pulmonary Artery
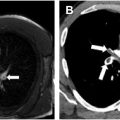
Proximal interruption of the pulmonary artery (PIPA) is a rare congenital anomaly with an absent or blind-ending mediastinal portion of the pulmonary artery and normally developed intrapulmonary pulmonary arteries. Pulmonary arterial supply is through collaterals from bronchial arteries and transpleural branches of the intercostal, internal mammary, subclavian, or brachiocephalic arteries. Most patients develop symptoms related to PIPA including recurrent pulmonary infections, hemoptysis, dyspnea, and pulmonary hypertension. Early detection of PIPA is important as it may offer the opportunity for surgical repair. Radiographic findings include unilateral absence of normal hilar vascular density, decreased pulmonary vascular markings, and mild hypoplasia of the affected lung with mediastinal shift to the affected side. , On CT, the abnormal pulmonary artery terminates within 1 cm of its origin from the main pulmonary artery ( Fig. 4 A–C ).

Pulmonary hypertension
Pulmonary arterial pressure equals systemic arterial pressure in utero , but falls rapidly after birth, reaching adult values by 3 months. After 3 months of age, pulmonary hypertension (PH) is defined as a mean pulmonary arterial pressure of greater than 20 mm Hg, a definition established at the 6th World Symposium on Pulmonary Hypertension (WSPH) and adopted by the Pediatric Task Force of the 6th WSPH
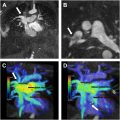
PH in children can begin at any age and as in adults can be due to many different etiologies. However, the distribution of PH etiologies in children is substantially different than in adults, with a greater predominance of idiopathic pulmonary arterial hypertension (PAH) as well as PH due to congenital heart disease (CHD) and developmental lung disease, and individual cases of PH in children are often associated with multiple etiologies. , In children, PH is currently classified according to the classification system established at the 6th WSPH. Children with PH have a high prevalence of genetic disorders. For example, variants in the TBX4 gene are associated with neonatal diffuse developmental lung disorders, familial or sporadic childhood PAH, and small patella syndrome ( Fig. 5 ). Additional genetic disorders resulting in PAH are described in the following sections.

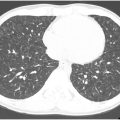
CT and cardiac MR imaging are important in the initial work-up of children with suspected PH. , CT is useful to assess for causes of secondary PH including vascular abnormalities, chronic thromboembolic disease, and parenchymal lung disease such as bronchopulmonary dysplasia (the most common lung disease associated with PH in children) ( Fig. 6 A, B ). , , , Cardiac MR imaging is useful to assess cardiac morphology and function. Nuclear medicine ventilation-perfusion imaging is also sometimes used to evaluate for evidence of chronic thromboembolic pulmonary hypertension. ,

Pulmonary vascular disease associated with genetic conditions
Williams Syndrome
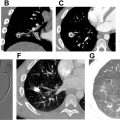
Williams syndrome is an autosomal dominant syndrome typically associated with a de novo chromosome 7q11.23 deletion encompassing the ELN gene that encodes the protein elastin. In addition to cognitive impairment, distinctive “elfin” facial features, and hypercalcemia in infancy, the syndrome is associated with cardiovascular abnormalities including supravalvular aortic stenosis and peripheral or branch pulmonary artery stenosis related to deficient circumferential arterial growth. Pulmonary artery stenosis occurs in approximately 60% of patients presenting in the first year of life and may be amenable to surgical pulmonary artery reconstruction but tends to progressively improve with age ( Fig. 7 A–D ). Pulmonary arterial diverticula at the bifurcation of the main pulmonary artery may also be observed.

Alagille Syndrome
Alagille syndrome is an autosomal dominant syndrome caused by mutations in the JAG1 gene encoding the Jagged 1 ligand in the Notch signaling pathway, resulting in bile duct paucity, butterfly vertebrae, ocular and cardiovascular abnormalities, most commonly pulmonary artery stenosis. On CTA, the stenoses most often involve the proximal left pulmonary artery and lobar and segmental pulmonary artery branch points. Associated congenital heart disease, most commonly tetralogy of Fallot, is associated with increased morbidity and mortality.
BMPR2-Associated Familial Pulmonary Arterial Hypertension
Germline mutations of the BMPR2 gene encoding the bone morphogenetic protein receptor type 2 transforming growth factor beta receptor are the predominant cause of familial PAH. Altered receptor signaling induces proliferation of smooth muscle cells and apoptosis-resistant endothelial cell clones in small pulmonary arteries, resulting in plexiform lesions at pulmonary arterial branchpoints and the origin of supernumerary vessels, appearing as ground-glass nodules with a central vessel on CT. These plexiform lesions are not pathognomonic of BMPR2 -associated familial PAH, and can also be observed in idiopathic PAH and in PAH in the setting of interstitial lung disease or congenital heart disease associated with low levels of BMPR2 expression or signaling.
SOX17-Associated Pulmonary Arterial Hypertension
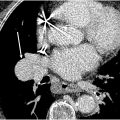
Autosomal dominant mutations in the SOX17 gene that encode SRY-box 17 involved in pulmonary vasculature formation and homeostasis are associated with severe sporadic or familial PAH. The PAH can be associated with life-threatening hemoptysis and cardiac septal defects, especially in young children. CT findings include dilated and tortuous pulmonary arteries, ground-glass halos around pulmonary arteries, and dilated bronchial arteries and subpleural vessels ( Fig. 8 A–C ).

Hereditary Hemorrhagic Telangiectasia
Hereditary hemorrhagic telangiectasia (HHT) is associated with pulmonary, hepatic, gastrointestinal, cerebrospinal, and mucocutaneous arteriovenous malformations (AVMs). Several genes have been implicated in the pathogenesis of HHT, including ENG , A CVRL1 (ALK1), SMAD4, HHT3 and HHT4 . On CT, pulmonary AVMs classically appear as an enhancing vascular tangle with feeding and draining vessels that may be amenable to endovascular therapy, although tiny AVMs may appear as solid or ground-glass nodules ( Fig. 9 ). In the setting of ACVRL1 mutations, PAH often precedes the manifestations of HHT.