Abstract
This chapter is focused on non-PET neoplasm imaging and radionuclide therapy. Gallium-67, thallium-201 chloride, technetium-99m sestamibi, pentetreotide, and antibody imaging are included. There is a discussion of breast-specific gamma camera imaging, as well as lymphoscintigrapy. Therapeutic agents for lymphoma, hepatoma, and hepatic metastases are presented.
Keywords
breast-specific gamma camera imaging, radiation necrosis, tumor receptor imaging, gallium-67 citrate, thallium-201 chloride, technetium-99m sestamibi, pentetreotide, lymphoscintigraphy, lymphoma antibody therapy, therapy with microspheres, DOTATATE
Chapter Outline
Radionuclide Tumor Antibody Imaging and Therapy
Treatment of Lymphoma With Radioimmunotherapy
Treatment of Neuroendocrine Tumors With Radiolabeled Somatostatin Analogs
Treatment of Hepatoma and Liver Metastases With Intravascular Microspheres
In this chapter, tumor imaging using conventional gamma camera techniques including single-photon emission computed tomography (SPECT) and SPECT/computed tomography (SPECT/CT) as well as less frequently employed or emerging radionuclide tumor therapies are addressed. The more commonly encountered entities of thyroid cancer and bone tumors and metastases are discussed in detail in Chapter 4 and Chapter 8 , respectively. Positron emission tomography (PET) imaging of neoplasms is discussed in Chapter 11 . The affinity of various tumors for specific radiopharmaceuticals is shown in Box 10.1 , and the relative value of various imaging procedures for different tumors is shown in Chapter 11 , Table 11.1 . Although some of these techniques have been largely supplanted by fluorine-18 fluorodeoxyglucose ( 18 F-FDG) PET/CT imaging, some are still useful in special settings.
Gallium-67 Citrate
Hodgkin disease
Non-Hodgkin lymphoma (especially high-grade)
Hepatoma
Bronchogenic carcinoma
Melanoma
Seminoma
Rhabdomyosarcoma
Thallium-201 Chloride
Gliomas (high-grade)
Thyroid carcinoma
Benign tumors (usually fade over 2 hours)
Osteosarcoma
Lymphoma (especially low-grade)
Kaposi sarcoma (gallium-negative)
Technetium-99m Sestamibi
Cancer metastases
Breast cancer
Parathyroid adenoma
Gliomas
Lymphoma
Thyroid
Indium-111 Pentetreotide
Amine precursor uptake and decarboxylation (APUD) cell tumors
Pancreatic islet cell
Pituitary adenoma
Pheochromocytoma
Neuroblastoma
Paraganglioma
Carcinoid
Gastrinoma
Vasoactive intestinal peptide-related tumors (VIPomas)
Medullary carcinoma of thyroid
Small cell lung cancer
Meningioma
Fluorine-18 Fluorodeoxyglucose
Most tumors (see Chapter 11 )
Head and neck cancer
Esophageal cancer
Non–small cell lung cancer
Melanoma
Lymphoma
Colorectal cancer
Breast cancer
Poorly differentiated neuroendocrine and thyroid tumors
Iodine-123 or -131 Sodium Iodide
Thyroid cancer
Iodine-123 or -131 Metaiodobenzylguanidine
Pheochromocytoma
Neuroblastoma
Paraganglioma
Monoclonal Antibodies
Lymphoma
During the past decade, new biotechnologic advances have spurred the development of increasingly sensitive and specific tumor imaging agents for use in both single-photon and positron imaging. As these agents have become available, they have spurred the concept of developing a drug or closely related group of drugs with high affinity to a particular tumor that can be labeled with a diagnostic imaging radionuclide to assess the location and extent of disease as well as separately labeled with a therapeutic radionuclide (such as a beta-emitter) to treat the neoplasm in a dosage commensurate with the tumor burden revealed by imaging. The success of treatment can be subsequently assessed using the diagnostic imaging version. This has been called “theranostics” (therapy + diagnosis). The simplest example is sodium iodide. Labeled with iodine-123, it is used diagnostically in differentiated thyroid cancer and its metastases. However, labeled with iodine-131, it becomes a therapeutic drug. More sophisticated compounds and their analogs are emerging for use with other tumors, such as somatostatin receptor radiopharmaceuticals for neuroendocrine malignancies. Tumor-imaging radiopharmaceuticals may be divided into two broad groups:
- •
Those designed to target specific tumor antigens, receptors, or metabolic processes, including monoclonal antibodies, peptides such as somatostatin (octreotide), and metaiodobenzylguanidine (MIBG).
- •
Those with nonspecific affinity for neoplastic tissue, including gallium-67 citrate ( 67 Ga), thallium-201 chloride ( 201 Tl), technetium-99m ( 99m Tc) sestamibi, and 18 F-FDG. These may be used to image a range of tumors in various organs.
Targeted Tumor Imaging
Neuropeptide Receptor Imaging
Neuropeptides constitute a family of highly potent substances that consist of only a few amino acids. They are synthesized and released primarily by the brain (hence the name neuropeptides ) and the gut and also by the endocrine system and lymphatic tissue. A number of neuropeptides and tissue receptors present as potential candidates for neuropeptide receptor imaging, including somatostatin.
Somatostatin (Octreotide) Receptor Imaging
Somatostatin is a naturally occurring neuropolypeptide that is synthesized and released by endocrine or nerve cells in various organs, especially the hypothalamus. It has a wide range of pharmacologic effects, including (as its name implies) the inhibition of secretion of growth hormone (somatotropin) by the pituitary gland. Because overexpression of somatostatin receptors occurs in numerous neuroendocrine and some non-neuroendocrine tumors, radiolabeled somatostatin analogs can be used to image a variety of primary tumors and their metastases. Neuroendocrine tumors are those derived from amine precursor uptake and decarboxylation (APUD) system cells, including carcinoid, pituitary adenomas, pancreatic islet cell neoplasms, medullary carcinoma of the thyroid, pheochromocytomas, neuroblastomas, paragangliomas, and small cell lung cancers.
Because endogenous somatostatin has a biologic half-life of only a few minutes, radiolabeled analogs with greater in vivo stability have been developed that have high specificity for somatostatin receptors, especially subtypes 2 and 5. This includes a commonly employed cyclic structural modification of octreotide, 111 In-labeled pentetreotide (OctreoScan), which has the additional advantage of reduced hepatobiliary excretion (2%) with clearance primarily through renal elimination (85% at 24 hours). Less gastrointestinal activity allows for better visualization of abdominal tumor sites, especially in the pancreas and duodenum. However, considerable liver activity may obscure small hepatic metastases. 68 Ga-labeled somatostatin analogs for PET/CT are now approved for use in the United States and have recently been recommended over pentetreotide for all indications. These PET radiopharmaceuticals are discussed in Chapter 11 .
Gamma camera imaging (planar and SPECT) is performed using an intravenous imaging dose of 6.0 mCi (222 MBq) of 111 In-pentetreotide. Adverse effects from pentetreotide occur in less than 1% of patients. Imaging is performed at 4 hours before the appearance of interfering bowel activity and again at 24 hours when tumor-to-background activity has increased, with 48-hour images acquired as needed to confirm a lesion suspected at 24 hours or when residual bowel or gallbladder activity is a problem. Planar images allow for survey of the whole body. SPECT or SPECT/CT images add to the sensitivity and specificity of the examination, especially in the upper abdomen, where interfering kidney, spleen, and often gallbladder activity may hamper imaging of the pancreas. SPECT may also be useful in better evaluating suspected liver metastases. A detailed imaging protocol is presented in Appendix E .
Prior to the procedure, patients should be well hydrated to enhance renal clearance. A laxative may be used to decrease interfering bowel activity. In patients with diarrheal syndromes or insulinomas, laxatives should be prescribed only after consultation with the referring physician.
Because of its ability to inhibit the secretion of hormones, stable octreotide is helpful in controlling symptoms in patients with hypersecretion syndromes associated with metastatic carcinoid tumors, gastrinomas, insulinomas, glucagonomas, and vasoactive intestinal peptide-related tumors (VIPomas). Although somatostatin receptor-positive tumors may be visualized in patients receiving stable octreotide therapy, it is preferable to discontinue the drug temporarily for 24 to 48 hours before administration of indium-111 ( 111 In)-pentetreotide.
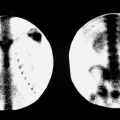
In a normal patient, 111 In-pentetreotide activity can be identified in the blood pool, normal thyroid gland, kidneys and bladder, liver, gallbladder, spleen, and, to a lesser degree, bowel on delayed images. The kidneys and spleen retain the most activity and therefore receive the highest absorbed doses. Because the radiopharmaceutical is in part retained by the renal parenchyma, the kidneys are seen even on delayed views ( Fig. 10.1 ).

In abnormal images, primary neoplasms or metastases present as foci of increased activity. Because somatostatin receptors are expressed in some nonneoplastic, chronic inflammatory processes, such as granulomatous lesions (sarcoidosis, tuberculosis), Crohn disease, ulcerative colitis, and rheumatoid arthritis, these entities may serve as potential sources of false-positive results.
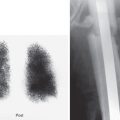
Indium-111-pentetreotide is primarily useful in evaluating neuroendocrine tumors, especially carcinoid (sensitivity, 85% to 95%) ( Fig. 10.2 ) and gastrinoma (sensitivity 75% to 93%) ( Fig. 10.3 ). A wider spectrum of tumors may be assessed with this radiopharmaceutical, however, including a number of nonendocrine solid tumors. ( Table 10.1 ) Although sensitive for evaluating pancreatic islet tumors, 111 In-pentetreotide is not useful in pancreatic carcinomas of exocrine origin because they do not express somatostatin receptors. The sensitivity of pentetreotide for imaging pheochromocytomas, neuroblastomas, and paragangliomas in extra-adrenal sites is more than 85%. Most of these are successfully imaged with radiolabeled somatostatin analogs with sensitivities approaching 80% to 100%. The absence of somatostatin receptor subtype 2, variable tumor differentiation, and variable receptor expression also influence tumor detectability. These factors may be pertinent in insulinoma (25% to 60% sensitivity) and medullary thyroid carcinoma (40% to 70% sensitivity). Some nonneuroendocrine tumors, including lymphomas, breast and lung carcinoma, gliomas (especially low-grade tumors), renal cell carcinoma, and meningiomas, show variable and unpredictable uptake. Whole-body gamma camera imaging provides cost-effective screening of patients with suspected or known somatostatin receptor-expressing tumors. The information obtained may disclose or confirm the presence of a lesion, detect metastases from a primary tumor, or characterize neuroendocrine conditions in which multicentric lesions may exist. In addition, because somatostatin receptor expression is seen more often in well-differentiated tumors, visualization may imply a more favorable prognosis. Patients with positive 111 In-pentetreotide images are also candidates for octreotide therapy because the documentation of somatostatin receptors provides a higher likelihood of controlling hormonal hypersecretion. Further, radionuclide therapy with radiolabeled somatostatin analogs, such as 177 Lu-DOTATATE, may be an option in some patients with octreotide-avid tumors.


| Neoplasm | Sensitivity | Comment |
|---|---|---|
| High Affinity | ||
| Carcinoid | 80–90% | Octreotide has higher sensitivity (> 80%) than MIBG in detecting primary and metastatic lesions |
| 70–100% | |
| Pituitary adenoma | 70–80% | Sensitivity depends on size, subtype, and degree of receptor expression Reported high for growth hormone and TSH-secreting tumors |
| 85–95% | MIBG has been reported to be more sensitive for benign PHEOs and 111 In-pentetreotide and 18 F-FDG more sensitive for malignant lesions and metastases |
| Neuroblastoma | 65–80% | Octreotide has lower sensitivity compared to MIBG |
| Meningioma | 90–100% | Rarely scanned for diagnostic purposes |
| Merkel cell Ca | 70–80% | Neuroendocrine skin lesions with high incidence of metastasis |
| Small cell lung Ca | 60–100% | Sensitivity for metastases is slightly less than for primary tumor |
| Variable Affinity | 50–75% | |
| Medullary thyroid Ca | 40–60% | Variation likely related to variation in degree of differentiation |
| Insulinoma | 25–60% | Lowest detectability of islet cell tumors |
| Medulloblastoma | 60–75% | |
| Low Affinity | ||
| More sensitive for low-grade astrocytomas than more aggressive lesions | |
| ||
| Graves disease, Graves ophthalmopathy, rheumatoid arthritis | |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree