Abstract
Background
Recent studies have shown that an increased number of axillary lymph node metastases is associated with non-visualized lymph nodes. The purpose of the study was to retrospectively analyze the incidence and characteristics of non-visualized sentinel lymph nodes (SLNs) in nodal metastases in breast cancer patients.
Methods
Consecutive women with breast cancer referred for lymphoscintigraphy from January 2021 to November 2022 were reviewed retrospectively. Findings from resected SLNs and non-SLNs and relevant histopathology were collected and analyzed.
Results
500 patients diagnosed with breast cancer were reviewed, excluding 93 patients due to neoadjuvant therapy, DCIS, recurrence, or incomplete clinical documentation. Of the 407 remaining patients, 108 patients were positive for axillary lymph node metastases (24 %) and were the focus of the study. Of this patient cohort, 38 patients (35 %) had non-detected SLNs by intraoperative gamma probe and 43 (40 %) had non-visualized SLNs by lymphoscintigraphy. There was statistically significant difference in primary tumor size (39.8 mm versus 28.9 mm), number of resected (6.9 ± 4.4 versus 4.6 ± 2.4) and positive (3.4 ± 2.2 versus 1.6 ± 1.3) lymph nodes, size (13.8 ± 6.1 mm versus 8.1 ± 4.5 mm), tumor grade and tumor stage between the SLN non-visualized and visualized groups. The multivariate logistic regression analysis showed that only lymph node size and number of lymph nodes resected were independent factors associated with SLN non-visualization.
Conclusions
We reported a high non-visualization rate of SLN in breast cancer patients with pathology-proven positive axillary nodes. The causes of the SLN non-visualization are not well understood and warrants further exploration.
1
Introduction
Breast cancer is the leading cause of cancer death despite early diagnosis via screening and surgery remains the major treatment . Lymphogenic dissemination is the primary route for breast tumor metastasis . The involvement of regional axillary lymph nodes is a crucial prognostic factor and has significant impact on tumor staging and treatment options . Sentinel lymph nodes (SLNs) are defined as the first nodes reached by tumor cells through the lymphatic channels. Thus, SLN mapping and biopsy have become the routine procedure used to assess the tumor status of the regional lymph nodes in breast cancer patients, particularly contributing to the development of less invasive surgical procedures .
While SLN dissection has proved to be effective and highly accurate with a low false negative rate [ , ], studies have shown that evidence of an increased number of axillary lymph node metastases is associated with non-visualized lymph nodes using preoperative sentinel node lymphoscintigraphy . Several hypotheses have been proposed to address the potential underlying pathophysiology of this observation [ , ]. Aside from technical and patient related factors, it has been speculated that massive lymphatic tumor invasion alters the homeostasis of the sentinel lymph nodes, which in turn impedes or obstructs normal lymph flow, ultimately preventing tracer migration . To date, it remains controversial whether or not the non-visualization of SLN is associated with a higher disease burden [ , ].
The purpose of the present study was to retrospectively analyze the incidence and characteristics of non-visualized sentinel lymph nodes for breast cancer and discuss the causes and potential clinical implications.
2
Materials and methods
2.1
Patient population
This single center, retrospective study was approved by the local Research Ethics Board. Five hundred women with breast cancer from January 1st 2021 to November 30th 2022 were reviewed in this study and preoperative lymphoscintigraphy data were collected. At our institute, breast cancer patients are routinely administered with radiopharmaceuticals preoperatively followed by lymphoscintigraphy mapping. During the same day, sentinel nodes are identified by an intraoperative gamma probe for resection. The number of resected lymph nodes and corresponding histopathology and radioactivity as assessed by intraoperative gamma probe were identified. Patients with disease recurrence, ductal carcinoma in situ (DCIS), neoadjuvant treatment before surgery and/or incomplete or missing operative notes after surgery were excluded. DCIS is a non-invasive or pre-invasive breast cancer and is considered stage 0. It usually does not metastasize beyond the breast and therefore it was excluded. In addition, there is insufficient evidence for lymphoscintigraphy in patients with DCIS .
Patients with pathological axillary lymph nodes were divided into nodal “ visualization ” and “ non-visualization ” groups, based on lymphoscintigraphy. The group definition also applied to the setting of lymph node resection in the operation room based on the detected nodal radioactivity by intraoperative gamma probe. This patient cohort with positive axillary lymph nodes is the focus of the current study. Note that the terms “hot” or “cold” node have been used interchangeably with the presence or absence of radioactivity, either detected by the probe or on imaging.
2.2
Lymphoscintigraphy
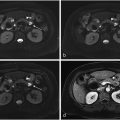
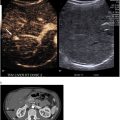
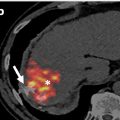
Lymphoscintigraphy was coordinated with the surgical department prior to the scheduled (same day) surgery. Patients received an average dose of 37 MBq (1 mCi) technetium-99 m ( 99 mTc) radiolabeled sulfur colloid filtered by a 0.22 µm filter diluted in 1 ml solution and mixed with 1 ml lidocaine in a total volume of 2 ml via a periareolar subcutaneous injection by experienced nuclear medicine technologists. Single-photon emission computed tomography (SPECT)/CT (low energy high resolution collimator, 360° coverage, step-and-shoot, contour orbit, 64 steps, 30 s per projection, 140 keV ± 7.5 %, 128 × 128; 130 keV, automatic mA) was obtained at 30 mins postinjection using a two-headed SPECT/CT system (Symbia-T SPECT-CT, Siemens Medical Solutions, USA). SPECT images were then reconstructed using a commercial software package (HybridRecon 2.1, Hermes Medical Solutions, Stockholm, Sweden) with attenuation, scatter, and resolution recovery corrections incorporating ordered subset expectation maximization with 4 iterations, 16 subsets and 0.89 mm full width at half maximum Gaussian post-filtering. Focal accumulations in at least one axillary lymph node were defined as SLN.
2.3
SLN dissection
The axillary SLN identification was performed using handheld gamma-ray detection probe and/or blue dye methods. Depending on the surgeons’ preference, blue dye injection was performed in some patients approximately 10 min before surgery to help visualize sentinel nodes. Lymph nodes exhibiting radioactivity uptake, blue dye uptake, or both were identified as SLN and excised. Clinically suspicious lymph nodes without radioactivity or blue dye were also excised. SLN that could not be detected using a gamma probe were classified as non-visualized SLN, in which case, lymph node dissection was performed. All excised lymph nodes were labeled with the information of radioactivity count and sent individually for histologic evaluation. The duration between radiotracer administration and the operation ranged from 2 to 6 h (median 273 min).
2.4
Histology
The dissected sentinel lymph nodes were fixed in formalin, embedded in paraffin, and cut in serial sections. Sections were stained with hematoxylin-eosin (H&E) for macrometastasis and immunohistochemical staining (IHC) for cytokeratin to detect micrometastasis (< 2 mm and > 0.1 mm in diameter). A SLN was considered positive if tumor cells were identified by H&E or IHC staining. For the current study, only SLNs with macrometastasis (> 2 mm) were considered positive.
2.5
Statistical analysis
Patient, tumor, and radiotracer characteristics were evaluated using descriptive statistics (mean±SD, and median(range)). Differences in parameters between non-visualized SLN and visualized SLN groups were assessed using Mann–Whitney U tests, as the distribution of the parameters were not normal (based on Shapiro-Wilk normality tests). Fisher’s exact tests were used to assess differences in proportion between groups for tumor grade and stage. Logistic-regression was used to examine the relationships between the different characteristics and SLN non-visualization. All statistical tests were two-tailed and a p value below 5 % was considered statistically significant.
3
Results
3.1
Tumor and intraoperative SLN characteristics
A total of 500 consecutive patients were reviewed and 93 patients were excluded from the study if they received neoadjuvant chemotherapy or had recurrent disease (49), were diagnosed with DCIS (31) or had incomplete or missing operative notes after surgery (13). Of the 407 remaining patients, 108 patients had positive axillary lymph node metastases (24 %). The patient group with biopsy proven axillary lymph node metastases ( n = 108) was the focus of the current study and the data were further analyzed for tumor/lymph node characteristics and image/probe findings. The breakdown of participants is shown in Fig. 1 .

The patient characteristics as well as tumor and nodal parameters are presented in Table 1 . Of the 108 patients with axillary lymph node metastases, 38 patients (35.5 %) had non-visualized pathological lymph nodes by intraoperative gamma probe, as determined from reviewing the operation and pathology reports. Statistically significant difference was identified in the primary tumor size (39.8 mm versus 28.9 mm, p = 0.027), number of resected lymph nodes (6.9 ± 4.4 versus 4.6 ± 2.4, p < 0.017), number of positive lymph nodes (3.4 ± 2.2 versus 1.6 ± 1.3, p < 0.001), and size of the largest resected axillary lymph node (13.8 ± 6.1 mm versus 8.1 ± 4.5 mm, p < 0.001) between the non-visualized and visualized groups ( Table 1 ). Based on the Fisher’s Exact test, there was statistically significant difference between the non-visualized and visualized groups in tumor grade ( p = 0.0432) and tumor stage ( p = 0.0025). The subsequent multivariate logistic regression analysis including patient age, number of nodes resected, node size, primary tumor size, tumor grade and clinical tumor stage showed that only lymph node size (odds ratio (standard error) 0.85 (0.06), p = 0.003) and number of lymph nodes resected (0.83 (0.09), p = 0.038) were independent factors associated with SLN non-visualization ( Table 2 ).
| Non-visualization group | Visualization group | ||||
|---|---|---|---|---|---|
| Number of patients | 38 | 70 | |||
| Mean ± SD number (%) ⁎⁎ | Median (range) | Mean ± SD | Median (range) | p values | |
| Age | 61.1 ± 11.5 | 64 (38,77) | 60.5 ± 12.1 | 62 (32,91) | 0.4196 |
| Stage | 0.0025 | ||||
| 2A | 4 (11 %) | 19 (27 %) | |||
| 2B | 15 (39 %) | 37 (53 %) | |||
| 3A | 13 (34 %) | 13 (19 %) | |||
| 3B/C | 6 (16 %) | 1 (1.4 %) | |||
| Tumor grade | 0.0432 | ||||
| 1 | 2 (5 %) | 15 (21 %) | |||
| 2 | 18 (47 %) | 34 (49 %) | |||
| 3 | 18 (47 %) | 21 (30 %) | |||
| Tumor size (mm) | 39.8 ± 28.3 | 30 (13,146) | 28.9 ± 15.6 | 25 (9.80) | 0.0270 |
| T ≤ 20 | 6 (16 %) | 20 (29 %) | 0.186 | ||
| 20 < T ≤ 50 | 25 (66 %) | 42 (60 %) | 0.156 | ||
| 50 < T | 7 (18 %) | 8 (11 %) | 0.045 | ||
| No. of resected nodes | 6.9 ± 0.44 | 5 (1,16) | 4.6 ± 2.4 | 4 (1,12) | 0.0170 |
| No. of positive nodes | 3.4 ± 2.2 | 3 (1,9) | 1.6 ± 1.3 | 1 (1,8) | <0.0001 |
| Lymph node size (mm) | 13.8 ± 6.1 | 12.5 (3.0, 27.0) | 8.1 ± 4.5 | 8.0 (2.2, 24.0) | <0.0001 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree