The prognosis for glioblastoma is poor despite optimal therapy with surgery, radiation, and chemotherapy. New therapies that improve survival and quality of life are needed. Research has increased our understanding of the molecular pathways important for gliomagenesis and disease progression. Novel agents have been developed against these targets, including receptor tyrosine kinases, intracellular signaling molecules, epigenetic abnormalities, and tumor vasculature and microenvironment. This article reviews novel therapies for glioblastoma, with an emphasis on targeted agents.
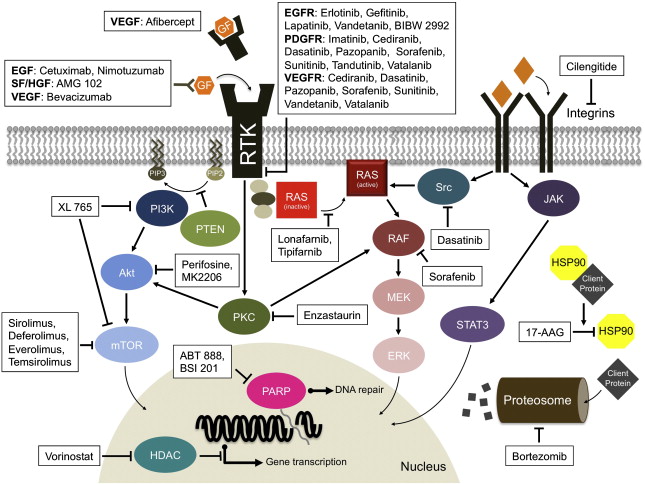
Glioblastoma multiforme (GBM) is the most common type of malignant primary brain tumor in adults. More than 10,000 new cases are diagnosed every year in the United States. Despite surgery, radiation, and chemotherapy, median survival is only 12 to 18 months. The demand for better therapies is high. In recent years, there has been significant progress in understanding the molecular pathways involved gliomagenesis and disease progression. Malignant transformation in gliomas is the result of sequential accumulation of genetic aberrations and deregulation of growth factor signaling pathways ( Fig. 1 ). Targeted therapies have been developed against these pathways.
Glioblastoma multiforme
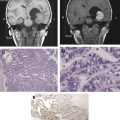
The incidence of GBM has increased over the past two decades primarily as a result of improved imaging. The diagnostic imaging modality of choice is magnetic resonance imaging (MRI) with gadolinium administration, which typically demonstrates an irregular ring-enhancing lesion with central necrosis and surrounding vasogenic edema. Definitive diagnosis is based on histology. GBM is a highly cellular tumor marked by mitoses, vascular proliferation, and pseudopallisading necrosis.
Glioblastomas can be separated into primary and secondary GBMs based on biologic and genetic differences. Primary GBMs occur typically in older patients and are characterized by epidermal growth factor receptor (EGFR) amplification and mutations, loss of heterozygosity of chromosome 10q, deletion of phosphatase and tensin homologue on chromosome 10 (PTEN), and p16 deletion. Secondary GBMs arise from lower grade gliomas over several years; these are much less common than primary GBMs and usually occur in younger patients. Secondary GBMs are characterized by p53 mutations, overexpression of platelet-derived growth factor receptor (PDGFR), abnormalities in the p16 and retinoblastoma pathways, loss of heterozygosity of chromosome 10q, and mutations of the isocitrate dehydrogenase 1 and 2 genes. Despite their genetic differences, primary and secondary GBMs are morphologically similar and likely share similar responses to conventional cytotoxic agents. On the other hand, these GBM subtypes may respond differently to targeted agents.
The standard of care for newly diagnosed GBM is based on results from a multicenter randomized trial. Although the addition of temozolomide (TMZ) to radiation therapy (RT) significantly prolonged survival in this study, the benefit was modest (median overall survival 12.1 months for RT alone versus 14.6 months for RT combined with TMZ). Most tumors eventually develop resistance to TMZ (median time to tumor progression is 6.9 months), and there is no standard chemotherapy for recurrent or progressive GBM because of unfavorable outcomes with currently available cytotoxic therapies.
Knowledge of GBM biology has revealed new potential therapeutic targets. Several novel agents against these molecular targets are now in clinical trials ( Table 1 ). Targeted therapy may provide greater specificity toward tumor cells with potentially less toxicity than conventional chemotherapy. The success of these agents in other systemic cancers demonstrates their potential in tumors with well-defined molecular targets. Even though the complexity of the molecular abnormalities in GBMs and the redundancy of the signaling pathways make it unlikely that single agents will achieve great success, there has been significant interest in this approach.
| Target | Mechanism of Action | Molecular Agent | Other Targets |
|---|---|---|---|
| Growth factors and growth factor receptors | |||
| EGFR | Reversible small-molecule inhibitor | Erlotinib (OSI-774, Tarceva) | |
| Gefitinib (ZD1839, Iressa) | |||
| Lapatinib (GW-572016, Tykerb) | HER2 | ||
| Vandetanib (ZD-6474, Zactima) | VEGFR | ||
| Irreversible small-molecule inhibitor | BIBW-2992 | HER2 | |
| PF-00299804 | HER2, HER4 | ||
| Monoclonal antibody | Cetuximab (Erbitux) | ||
| Nimotuzumab (h-R3, TheraCIM, TheraLOC) | |||
| EMD 55 900 | |||
| Iodine-125 labeled monoclonal antibody-425 | |||
| EGFRvIII | Peptide based vaccine | CDX-110 | |
| Monoclonal antibody | mAb 806 | ||
| SF/HGF | Monoclonal antibody | AMG-102 | |
| PDGFR | Small-molecule inhibitor | Cediranib (AZD-2171, Recentin) | VEGFR, c-Kit |
| Dasatinib (BMS-354825, Sprycel) | Src, Abl, VEGFR, Flt-3 | ||
| Imatinib mesylate (STI-571, Gleevec) | Bcr-Abl, c-Fms, c-Kit | ||
| Pazopanib (GW-786034) | VEGFR, c-Kit | ||
| Sorafenib (BAY-439006, Nexavar) | VEGFR, Raf, c-Kit | ||
| Sunitinib (SU-11248, Sutent) | VEGFR, c-Kit, Flt-3 | ||
| Tandutinib (MLN-518) | Flt-3, c-Kit | ||
| Vatalanib (PTK-787/AK-222584) | VEGFR, c-Kit, c-Fms | ||
| PDGFR-α | Monoclonal antibody | IMC-3G3 | |
| Intracellular signaling | |||
| Ras | Farnesyl transferase inhibitor | Lonafarnib (SCH-66336, Sarasar) Tipifarnib (R115777, Zanestra) | |
| Raf | Small-molecule inhibitor | Sorafenib (BAY-439006, Nexavar) | VEGFR, PDGFR, Flt-3, c-Kit, FGFR |
| mTOR | Small-molecule inhibitor | Ridaforolimus (AP-23573) | |
| Everolimus (RAD-001, Afinitor) | |||
| Sirolimus (Rapamycin, Rapamune) | |||
| Temsirolimus (CCI-779, Torisel) | |||
| XL-765 | PI3K | ||
| PI3K | Small-molecule inhibitor | XL-765 XL147 BKM 120 | mTOR |
| Akt | Alkylphospholipid | Perifosine (KRX-0401) | |
| Small-molecule inhibitor | MK2206 | ||
| PKC | Small-molecule inhibitor | Enzastaurin (LY-317615) | |
| Tumor vasculature | |||
| VEGF | Soluble decoy receptor | Afibercept (VEGF-Trap) | |
| Monoclonal antibody | Bevacizumab (Avastin) | ||
| VEGFR | Small-molecule inhibitor | Cediranib (AZD-2171, Recentin) | PDGFR, c-Kit |
| Dasatinib (BMS-354825, Sprycel) | Src, Abl, PDGFR, Flt-3 | ||
| Pazopanib (GW-786034) | PDGFR, c-Kit | ||
| Sorafenib (BAY-439006, Nexavar) | PDGFR, Raf, c-Kit | ||
| Sunitinib (SU-11248, Sutent) | PDGFR, c-Kit, Flt-3 | ||
| Vandetanib (ZD-6474, Zactima) | EGFR | ||
| Vatalanib (PTK-787/AK-222584) | PDGFR, c-Kit, c-Fms | ||
| XL184 | Met | ||
| Foretinib | Met, PDGFR | ||
| Adnectin | CT-332 (Angiocept) | ||
| Monoclonal antibody | IMC-1121B | ||
| Met | Small-molecule inhibitor | XL184 | VEGFR |
| Integrins | Small-molecule inhibitor | Cilengitide (EMD-121974) ATN-161 | |
| Cytokines | |||
| TGF-β | Antisense oligonucleotide | AP 12009 | |
| Protein turnover | |||
| Proteasome inhibitor | Bortezomib (MLN-341, Velcade) | ||
| Chromatin remodeling | |||
| HDAC inhibitor | Vorinostat (SAHA, Zolinza) | ||
| Panobinostat (LBH589) | |||
| Heat shock proteins | |||
| HSP90 inhibitor | Tanespimycin (17-AAG, KOS-953) | ||
| DNA repair | |||
| PARP inhibitor | ABT-888 BSI-201 | ||
Cellular signal transduction pathway
Tyrosine Kinase Inhibitors
Tyrosine kinases are a group of protein kinases that are critical to many signal transduction pathways involved in cell proliferation, growth, survival, adhesion, motility, and differentiation. This group includes receptor tyrosine kinases (RTKs), which are transmembrane proteins containing an extracellular binding domain that binds ligands and an intracellular kinase domain that activates intracellular signaling pathways.
Epidermal growth factor
The EGFR pathway is an attractive target because it is frequently dysregulated in GBMs through overexpression, amplification, or activating mutations. EGFR dysregulation results in aberrant signaling which, in turn, is associated with increased proliferation, motility, and survival of tumor cells in vitro. The most common EGFR mutation, found in 20% to 30% of GBMs, is EGFRvIII, which causes constitutive activation and phosphorylation of the receptor without ligand binding. Several approaches exist for targeting EGFR including small-molecule tyrosine kinase inhibitors (TKIs), monoclonal antibodies (mAbs), and vaccines.
The most extensively studied TKIs in GBM are erlotinib and gefitinib. Both are oral, low molecular weight, reversible inhibitors of tyrosine kinase activity associated with EGFR. When combined with RT in newly diagnosed GBM patients, the addition of these agents did not prolong 6-month progression-free survival (6M-PFS) or overall survival (OS) in multicenter trials as compared with standard therapy. In several phase 2 clinical trials of recurrent high-grade gliomas, neither gefitinb nor erlotinib monotherapy demonstrated any significant survival benefit as compared with historical controls. Clinical activity was modest in the GBM subset, with radiographic responses (RR) ranging from 0% to 13% for gefitinib and 0% to 26% for erlotinib, and with 6M-PFS ranging from 9% to 13% for gefitinib and 0% to 26% for erlotinib. Small subgroups of patients had durable responses. Both TKIs were generally well tolerated with rash, diarrhea, and fatigue as the most common side effects. The combination of EGFR inhibitors with other therapies is discussed later in this article.
Lapatinib, which inhibits ErbB2/HER2 (human epidermal growth factor receptor 2) as well as EGFR, has also been evaluated in recurrent glioblastomas. This agent achieves therapeutic concentrations in gliomas but has limited activity.
Although subsets of GBM patients have sustained responses to reversible TKIs that target EGFR, the studies to date have been largely disappointing. Potential reasons for lack of response include poor blood-brain barrier penetration, insufficient local tumor concentrations, coactivation of multiple TKIs, redundant signaling pathways, and resistance. Irreversible EGFR inhibitors, such as BIBW 2992 and PF-00299804, could have better efficacy in GBM than gefitinib or elotinib due to increased potency and in the case of PF-00299804, better brain concentration. This newer class of EGFR inhibitors has been shown to circumvent mechanisms of response to gefitinib or erlotinib in non–small cell lung cancer cells.
Attempts to define subsets of erlotinib or gefitinib responders have also been met with limited success. The activating mutations found in EGFR in non–small cell lung cancer that increase response to EGFR inhibitors are not present in GBM. Initial molecular studies suggested that gefitinib and erlotinib were more effective in tumors with EGFRvIII mutations and intact PTEN. However, recent studies failed to validate these results.
Monoclonal antibodies (mAb) as well as vaccines that target EGFR are currently under investigation in GBM. These therapies have been well tolerated in early clinical trials. Results from a phase 1/2 study combining nimotuzumab (a humanized anti-EGFR mAb) with radiotherapy in patients were favorable ( Table 2 ). Both nimotuzumab and cetuximab, a chimeric anti-EGFR human-mouse mAb, are now being studied in combination with radiation and temozolomide as upfront GBM therapies. Preliminary results from a phase 2 clinical trial suggest that the addition of CDX-110, a peptide based EGFRvIII vaccine, to standard therapy prolongs survival in patients with newly diagnosed GBM (see Table 2 ). However, patients were required to have gross total resections as well as EGFRvIII positivity by immunohistochemistry in order to be eligible for this trial.
| Therapy | Trial Phase | Patient Population | Trial Number and Sponsor | Status |
|---|---|---|---|---|
| ABT-888, RT, TMZ | Multicenter phase 1/2 study | GBM | NCT00770471, ABTC | Ongoing |
| Aflibercept, RT, TMZ | Multicenter phase 1/2 study | High-grade glioma | NCT00650923, ABTC | Ongoing |
| Bevacizumab, irinotecan, RT vs bevacizumab, TMZ, RT | Multicenter randomized phase 2 study | GBM | NCT00817284, Rigshospitalet | Ongoing |
| Bevacizumab, RT, TMZ vs RT, TMZ | Multicenter randomized phase 3 study | GBM, gliosarcoma | NCT00884741, RTOG | Ongoing |
| Bevacizumab, RT, TMZ vs RT, TMZ | Multicenter randomized phase 3 study | GBM | NCT00943826 Roche | Ongoing |
| Bevacizumab, erlotinib, TMZ | Single-center phase 2 study | GBM with stable disease following concurrent RT and TMZ | NCT00525525, University of California at San Francisco | Ongoing |
| BSI-201, RT, TMZ | Multicenter Phase 1/2 study | High-grade glioma | NCT00687765, ABTC | Ongoing |
| Cediranib, RT, TMZ | Multicenter phase 1/2 study | GBM | NCT00662506, Massachusetts General Hospital | Ongoing |
| Cetuximab, RT, TMZ | Single-center phase 1/2 study | GBM | NCT00311857, University of Heidelberg | Ongoing |
| CDX-110, RT, TMZ | Multicenter phase 2 study | GBM with gross total resection and EGFRvIII positivity by IHC | NCT00458601, Pfizer | Preliminary results : median OS 33.3 months, median PFS 16.6 months |
| Cilengitide, RT, TMZ vs RT, TMZ | Multicenter randomized phase 3 study | GBM with methylated MGMT | NCT00689221, EORTC | Ongoing |
| Cilengitide, RT, TMZ | Single-center phase 2 study | GBM with unmethylated MGMT | NCT00813943, Merck Serono | Ongoing |
| Cilengitide, RT, TMZ | Multicenter phase 1/2 study | GBM | NCT00085254, Merck Serono | Ongoing |
| CT-322, RT, TMZ | Multicenter phase 1 study | GBM | NCT00768911, Adnexus | Ongoing |
| Dasatinib, RT, TMZ | Mutlicenter NCCTG phase 1/2 study | GBM | NCT00869401, NCCTG | Not yet recruiting |
| Enzastaurin, RT | Multicenter phase 2 study | GBM with unmethylated MGMT | NCT00509821, Eli Lilly and Company | Ongoing |
| Enzastaurin, RT, TMZ | Single-center phase 1/2 study | GBM, gliosarcoma | NCT00402116, Eli Lilly and Company, University of California at San Francisco | Ongoing |
| Erlotinib, RT, TMZ | Multicenter phase 1/2 study | GBM | NCT00039494, NCCTG | Results : median OS 15.3 mo, median PFS 7.2 mo |
| Erlotinib, RT, TMZ | Single-center phase 2 study | GBM, gliosarcoma | NCT00187486, University of California at San Francisco | Results : median OS 19.3 mo, median PFS 8.2 mo |
| Erlotinib, RT, TMZ | Single-center phase 2 study | GBM | NCT00274833, Case Comprehensive Cancer Center | Ongoing |
| Everolimus, RT, TMZ | Multicenter phase 1/2 study | GBM | NCT00553150, NCCTG | Ongoing |
| Everolimus, TMZ | Multicenter phase 1 study | GBM with stable disease following concurrent RT and TMZ | NCT00387400, National Cancer Institute of Canada | Ongoing |
| Gefitinib, RT | Multicenter phase 1/2 study | GBM | NCT00052208, RTOG | Preliminary results : median OS 11.0 mo, median PFS 5.1 mo |
| Nimotuzumab, RT | Multicenter phase 1/2 study | High-grade glioma | National Center for Clinical Trials (Cuba) | Results : median OS 17.47 mo for GBM subset, RR 31.3% for GBM subset |
| Nimotuzumab, RT, TMZ | Multicenter randomized phase 3 study | GBM | NCT00753246, Oncoscience AG | Ongoing |
| Sorafenib, RT, TMZ | Single-center phase 1/2 study in GBM | GBM, gliosarcoma | NCT00734526, MD Anderson Cancer Center | Ongoing |
| Sorafenib, TMZ following RT and TMZ | Multicenter phase 2 study | GBM | NCT00544817, SCRI Oncology Research Consortium | Ongoing |
| Temsirolimus, RT, TMZ | Multicenter phase 1 study | GBM | NCT00316849, NCCTG | Ongoing |
| Tipifarnib, RT | Multicenter phase 1/2 study | GBM | NCT00209989, Institut Claudius Regaud | Ongoing |
| Tipifarnib (prior to RT) | Multicenter phase 2 study | GBM with residual enhancing disease | NCT00058097, NABTT CNS Consortium | Results median OS 7.7 mo, RR 0, study stopped early due to progression of disease in 48% |
| Tipifarnib, RT, TMZ | Multicenter NABTC phase 1 study in GBM | GBM, Gliosarcoma | NCT00049387, NABTC | Ongoing |
| Vandetanib, RT, TMZ | Multicenter phase 1/2 study in GBM | GBM, gliosarcoma | NCT00441142, Dana Farber Cancer Institute | Ongoing |
| Vatalanib, RT, TMZ vs RT, TMZ | Multicenter randomized phase 1/2 study | GBM | NCT00128700, EORTC | Phase 1 study completed, phase 2 study ongoing |
| Vatalanib, RT, TMZ | Multicenter phase 1 study | GBM on enzyme-inducing antiepiletics | NCT00385853, Massachusetts General Hospital | Ongoing |
| Vorinostat, RT, TMZ | Multicenter phase 1/2 study | GBM | NCT00731731, NCCTG and ABTC | Ongoing |
Scatter factor/hepatocyte growth factor
Scatter factor/hepatocyte growth factor (SF/HGF) and its tyrosine kinase receptor c-Met play a role in cell growth, cell motility, morphogenesis, and angiogenesis. HGF and c-Met are overexpressed in gliomas, and expression levels correlate with grade and the degree of malignancy. AMG-102 is a fully human monoclonal antibody that selectively targets SF/HGF. A phase 2 study of AMG-102 in recurrent GBM was recently completed but failed to produce any benefit. It is unclear whether the lack of activity was related to the inability of AMG-102 to pass through the blood-tumor barrier or whether inhibiting HGF alone was insufficient. A recent study suggests that the combination of EGFR inhibitors and Met inhibitors may be more effective than either agent alone in PTEN-null GBM tumors. Met TKIs such as XL184 are in clinical trial in GBM.
Platelet-derived growth factor
Platelet-derived growth factors (PDGFs) are a pleiotropic family of peptides that signal through PDGFR to stimulate cellular functions including growth, proliferation, and differentiation. PDGF and PDGFR are overexpressed in gliomas and are associated with more malignant tumors. Blocking PDGF-mediated phosphorylation causes either apoptosis or growth inhibition in glioma cell lines. PDGF-C also induces angiogenic activity via activation of PDGFR-α and -β receptors as well as upregulation of vascular endothelial growth factor (VEGF).
Imatinib mesylate, an inhibitor of PDGFR-α, PDGFR-β, Bcr-Abl, c-Fms, and c-Kit tyrosine kinases, demonstrated activity in preclinical models of glioma. However, in two multicenter phase 2 clinical trials, imatinib monotherapy was not associated with clinically useful activity in GBM, with RR of 3% to 13% and 6M-PFS of 3% to 16%. Results were also disappointing in a multicenter phase 3 trial of imatinib plus hydroxyurea (a ribonucleoside diphosphate reductase inhibitor) versus hydroxyurea monotherapy in progressive GBM. Radiographic response rates by central review and 6M-PFS were similar in both arms. One explanation for the lack of efficacy is that imatinib is a substrate for the P-glycoprotein efflux pump, which limits its passage over an intact blood-brain barrier. Two second-generation PDGFR inhibitors with improved central nervous system penetration, tandutinib and dasatinib, are in clinical trials for recurrent high-grade glioma, as is IMC-3G3, an antibody against PDGFR-α.
Cellular signal transduction pathway
Tyrosine Kinase Inhibitors
Tyrosine kinases are a group of protein kinases that are critical to many signal transduction pathways involved in cell proliferation, growth, survival, adhesion, motility, and differentiation. This group includes receptor tyrosine kinases (RTKs), which are transmembrane proteins containing an extracellular binding domain that binds ligands and an intracellular kinase domain that activates intracellular signaling pathways.
Epidermal growth factor
The EGFR pathway is an attractive target because it is frequently dysregulated in GBMs through overexpression, amplification, or activating mutations. EGFR dysregulation results in aberrant signaling which, in turn, is associated with increased proliferation, motility, and survival of tumor cells in vitro. The most common EGFR mutation, found in 20% to 30% of GBMs, is EGFRvIII, which causes constitutive activation and phosphorylation of the receptor without ligand binding. Several approaches exist for targeting EGFR including small-molecule tyrosine kinase inhibitors (TKIs), monoclonal antibodies (mAbs), and vaccines.
The most extensively studied TKIs in GBM are erlotinib and gefitinib. Both are oral, low molecular weight, reversible inhibitors of tyrosine kinase activity associated with EGFR. When combined with RT in newly diagnosed GBM patients, the addition of these agents did not prolong 6-month progression-free survival (6M-PFS) or overall survival (OS) in multicenter trials as compared with standard therapy. In several phase 2 clinical trials of recurrent high-grade gliomas, neither gefitinb nor erlotinib monotherapy demonstrated any significant survival benefit as compared with historical controls. Clinical activity was modest in the GBM subset, with radiographic responses (RR) ranging from 0% to 13% for gefitinib and 0% to 26% for erlotinib, and with 6M-PFS ranging from 9% to 13% for gefitinib and 0% to 26% for erlotinib. Small subgroups of patients had durable responses. Both TKIs were generally well tolerated with rash, diarrhea, and fatigue as the most common side effects. The combination of EGFR inhibitors with other therapies is discussed later in this article.
Lapatinib, which inhibits ErbB2/HER2 (human epidermal growth factor receptor 2) as well as EGFR, has also been evaluated in recurrent glioblastomas. This agent achieves therapeutic concentrations in gliomas but has limited activity.
Although subsets of GBM patients have sustained responses to reversible TKIs that target EGFR, the studies to date have been largely disappointing. Potential reasons for lack of response include poor blood-brain barrier penetration, insufficient local tumor concentrations, coactivation of multiple TKIs, redundant signaling pathways, and resistance. Irreversible EGFR inhibitors, such as BIBW 2992 and PF-00299804, could have better efficacy in GBM than gefitinib or elotinib due to increased potency and in the case of PF-00299804, better brain concentration. This newer class of EGFR inhibitors has been shown to circumvent mechanisms of response to gefitinib or erlotinib in non–small cell lung cancer cells.
Attempts to define subsets of erlotinib or gefitinib responders have also been met with limited success. The activating mutations found in EGFR in non–small cell lung cancer that increase response to EGFR inhibitors are not present in GBM. Initial molecular studies suggested that gefitinib and erlotinib were more effective in tumors with EGFRvIII mutations and intact PTEN. However, recent studies failed to validate these results.
Monoclonal antibodies (mAb) as well as vaccines that target EGFR are currently under investigation in GBM. These therapies have been well tolerated in early clinical trials. Results from a phase 1/2 study combining nimotuzumab (a humanized anti-EGFR mAb) with radiotherapy in patients were favorable ( Table 2 ). Both nimotuzumab and cetuximab, a chimeric anti-EGFR human-mouse mAb, are now being studied in combination with radiation and temozolomide as upfront GBM therapies. Preliminary results from a phase 2 clinical trial suggest that the addition of CDX-110, a peptide based EGFRvIII vaccine, to standard therapy prolongs survival in patients with newly diagnosed GBM (see Table 2 ). However, patients were required to have gross total resections as well as EGFRvIII positivity by immunohistochemistry in order to be eligible for this trial.
| Therapy | Trial Phase | Patient Population | Trial Number and Sponsor | Status |
|---|---|---|---|---|
| ABT-888, RT, TMZ | Multicenter phase 1/2 study | GBM | NCT00770471, ABTC | Ongoing |
| Aflibercept, RT, TMZ | Multicenter phase 1/2 study | High-grade glioma | NCT00650923, ABTC | Ongoing |
| Bevacizumab, irinotecan, RT vs bevacizumab, TMZ, RT | Multicenter randomized phase 2 study | GBM | NCT00817284, Rigshospitalet | Ongoing |
| Bevacizumab, RT, TMZ vs RT, TMZ | Multicenter randomized phase 3 study | GBM, gliosarcoma | NCT00884741, RTOG | Ongoing |
| Bevacizumab, RT, TMZ vs RT, TMZ | Multicenter randomized phase 3 study | GBM | NCT00943826 Roche | Ongoing |
| Bevacizumab, erlotinib, TMZ | Single-center phase 2 study | GBM with stable disease following concurrent RT and TMZ | NCT00525525, University of California at San Francisco | Ongoing |
| BSI-201, RT, TMZ | Multicenter Phase 1/2 study | High-grade glioma | NCT00687765, ABTC | Ongoing |
| Cediranib, RT, TMZ | Multicenter phase 1/2 study | GBM | NCT00662506, Massachusetts General Hospital | Ongoing |
| Cetuximab, RT, TMZ | Single-center phase 1/2 study | GBM | NCT00311857, University of Heidelberg | Ongoing |
| CDX-110, RT, TMZ | Multicenter phase 2 study | GBM with gross total resection and EGFRvIII positivity by IHC | NCT00458601, Pfizer | Preliminary results : median OS 33.3 months, median PFS 16.6 months |
| Cilengitide, RT, TMZ vs RT, TMZ | Multicenter randomized phase 3 study | GBM with methylated MGMT | NCT00689221, EORTC | Ongoing |
| Cilengitide, RT, TMZ | Single-center phase 2 study | GBM with unmethylated MGMT | NCT00813943, Merck Serono | Ongoing |
| Cilengitide, RT, TMZ | Multicenter phase 1/2 study | GBM | NCT00085254, Merck Serono | Ongoing |
| CT-322, RT, TMZ | Multicenter phase 1 study | GBM | NCT00768911, Adnexus | Ongoing |
| Dasatinib, RT, TMZ | Mutlicenter NCCTG phase 1/2 study | GBM | NCT00869401, NCCTG | Not yet recruiting |
| Enzastaurin, RT | Multicenter phase 2 study | GBM with unmethylated MGMT | NCT00509821, Eli Lilly and Company | Ongoing |
| Enzastaurin, RT, TMZ | Single-center phase 1/2 study | GBM, gliosarcoma | NCT00402116, Eli Lilly and Company, University of California at San Francisco | Ongoing |
| Erlotinib, RT, TMZ | Multicenter phase 1/2 study | GBM | NCT00039494, NCCTG | Results : median OS 15.3 mo, median PFS 7.2 mo |
| Erlotinib, RT, TMZ | Single-center phase 2 study | GBM, gliosarcoma | NCT00187486, University of California at San Francisco | Results : median OS 19.3 mo, median PFS 8.2 mo |
| Erlotinib, RT, TMZ | Single-center phase 2 study | GBM | NCT00274833, Case Comprehensive Cancer Center | Ongoing |
| Everolimus, RT, TMZ | Multicenter phase 1/2 study | GBM | NCT00553150, NCCTG | Ongoing |
| Everolimus, TMZ | Multicenter phase 1 study | GBM with stable disease following concurrent RT and TMZ | NCT00387400, National Cancer Institute of Canada | Ongoing |
| Gefitinib, RT | Multicenter phase 1/2 study | GBM | NCT00052208, RTOG | Preliminary results : median OS 11.0 mo, median PFS 5.1 mo |
| Nimotuzumab, RT | Multicenter phase 1/2 study | High-grade glioma | National Center for Clinical Trials (Cuba) | Results : median OS 17.47 mo for GBM subset, RR 31.3% for GBM subset |
| Nimotuzumab, RT, TMZ | Multicenter randomized phase 3 study | GBM | NCT00753246, Oncoscience AG | Ongoing |
| Sorafenib, RT, TMZ | Single-center phase 1/2 study in GBM | GBM, gliosarcoma | NCT00734526, MD Anderson Cancer Center | Ongoing |
| Sorafenib, TMZ following RT and TMZ | Multicenter phase 2 study | GBM | NCT00544817, SCRI Oncology Research Consortium | Ongoing |
| Temsirolimus, RT, TMZ | Multicenter phase 1 study | GBM | NCT00316849, NCCTG | Ongoing |
| Tipifarnib, RT | Multicenter phase 1/2 study | GBM | NCT00209989, Institut Claudius Regaud | Ongoing |
| Tipifarnib (prior to RT) | Multicenter phase 2 study | GBM with residual enhancing disease | NCT00058097, NABTT CNS Consortium | Results median OS 7.7 mo, RR 0, study stopped early due to progression of disease in 48% |
| Tipifarnib, RT, TMZ | Multicenter NABTC phase 1 study in GBM | GBM, Gliosarcoma | NCT00049387, NABTC | Ongoing |
| Vandetanib, RT, TMZ | Multicenter phase 1/2 study in GBM | GBM, gliosarcoma | NCT00441142, Dana Farber Cancer Institute | Ongoing |
| Vatalanib, RT, TMZ vs RT, TMZ | Multicenter randomized phase 1/2 study | GBM | NCT00128700, EORTC | Phase 1 study completed, phase 2 study ongoing |
| Vatalanib, RT, TMZ | Multicenter phase 1 study | GBM on enzyme-inducing antiepiletics | NCT00385853, Massachusetts General Hospital | Ongoing |
| Vorinostat, RT, TMZ | Multicenter phase 1/2 study | GBM | NCT00731731, NCCTG and ABTC | Ongoing |
Scatter factor/hepatocyte growth factor
Scatter factor/hepatocyte growth factor (SF/HGF) and its tyrosine kinase receptor c-Met play a role in cell growth, cell motility, morphogenesis, and angiogenesis. HGF and c-Met are overexpressed in gliomas, and expression levels correlate with grade and the degree of malignancy. AMG-102 is a fully human monoclonal antibody that selectively targets SF/HGF. A phase 2 study of AMG-102 in recurrent GBM was recently completed but failed to produce any benefit. It is unclear whether the lack of activity was related to the inability of AMG-102 to pass through the blood-tumor barrier or whether inhibiting HGF alone was insufficient. A recent study suggests that the combination of EGFR inhibitors and Met inhibitors may be more effective than either agent alone in PTEN-null GBM tumors. Met TKIs such as XL184 are in clinical trial in GBM.
Platelet-derived growth factor
Platelet-derived growth factors (PDGFs) are a pleiotropic family of peptides that signal through PDGFR to stimulate cellular functions including growth, proliferation, and differentiation. PDGF and PDGFR are overexpressed in gliomas and are associated with more malignant tumors. Blocking PDGF-mediated phosphorylation causes either apoptosis or growth inhibition in glioma cell lines. PDGF-C also induces angiogenic activity via activation of PDGFR-α and -β receptors as well as upregulation of vascular endothelial growth factor (VEGF).
Imatinib mesylate, an inhibitor of PDGFR-α, PDGFR-β, Bcr-Abl, c-Fms, and c-Kit tyrosine kinases, demonstrated activity in preclinical models of glioma. However, in two multicenter phase 2 clinical trials, imatinib monotherapy was not associated with clinically useful activity in GBM, with RR of 3% to 13% and 6M-PFS of 3% to 16%. Results were also disappointing in a multicenter phase 3 trial of imatinib plus hydroxyurea (a ribonucleoside diphosphate reductase inhibitor) versus hydroxyurea monotherapy in progressive GBM. Radiographic response rates by central review and 6M-PFS were similar in both arms. One explanation for the lack of efficacy is that imatinib is a substrate for the P-glycoprotein efflux pump, which limits its passage over an intact blood-brain barrier. Two second-generation PDGFR inhibitors with improved central nervous system penetration, tandutinib and dasatinib, are in clinical trials for recurrent high-grade glioma, as is IMC-3G3, an antibody against PDGFR-α.
Intracellular signaling kinases
Serine-Threonine Kinases
The serine-threonine kinases are a family of enzymes that phosphorylate serine and threonine residues. Many of these kinases serve as intermediates in important signaling pathways.
PI3K/Akt pathway inhibitors
Phosphatidylinositol 3-kinase/AKT (PI3K/Akt) is a signaling pathway important for cell growth and survival. The pathway is activated by several RTKs, including EGFR and PDGFR, and is inhibited by PTEN. Activation of the PI3K-Akt pathway is associated with poor prognosis in glioma patients and with more aggressive tumors. In vivo studies suggest that PI3K inhibitors sensitize GBM cells to apoptosis. Several PI3K and Akt inhibitors are in development or early clinical trials. XL765, an inhibitor of PI3K and mTOR, is currently in a phase 1 clinical trial in combination with temozolomide for patients with high-grade glioma. Akt inhibitors undergoing evaluation in high-grade glioma include perifosine and MK2206.
PI3K/Akt signaling leads to downstream activation of several targets including the mammalian target of rapamycin (mTOR). The mTOR inhibitor sirolimus (rapamycin) and its analogues temsirolimus, everolimus, and ridaforolimus are the most clinically advanced PI3K/Akt pathway inhibitors. Despite promising results from preclinical studies, temsirolimus monotherapy was not clinically active in recurrent GBM in two multicenter phase 2 clinical trials. Radiographic response rates were 0% and 5% while 6M-PFS rates were 2% and 8%. One possible explanation for the lack of efficacy is that rapamycin inhibition of TOR complex 1 (TORC1) disrupts the negative feedback loop on the Akt pathway, which may lead paradoxically to Akt activation. An Akt inhibitor, such as perifosine or MK2206, or a combined PI3K/mTOR inhibitor, such as XL765, may ultimately prove more favorable. Studies combining mTOR inhibitors with other targeted agents are discussed later in this article.
Ras-mitogen activated protein kinase pathway inhibitors
The mitogen-activated protein kinase (MAPK) pathway is involved in important signaling cascades that regulate normal cell proliferation, survival, and differentiation. The pathway is triggered by several RTKs, including EGFR and vascular endothelial growth factor receptor (VEGFR), and results in downstream activation of Ras, a guanine nucleotide triphosphatase. Ras then transmits signals to MAPK, PI3K, and other signaling pathways.
Several posttranslational modifications of Ras are necessary for proper functioning. One of these modifications is the addition of a farnesyl group, which enables Ras to translocate from the cytoplasm to the cell membrane. Farnesyl transferase inhibitors (FTIs), such as tipifarnib and lonafarnib, are designed to block farnesylation and ultimately prevent proper functioning of Ras. These agents demonstrated activity in human glioma cell lines, but results from clinical trials have been disappointing. Both tipifarnib and lonafarnib are now being studied in combination with TMZ for high-grade gliomas.
The Raf serine/threonine kinases are the main downstream effectors of Ras in the MAPK pathway. Inhibiting at the level of Raf may be more efficacious because it is a key element situated at the convergence of several redundant signaling pathways. Activating Raf mutations have been found in several malignancies, including GBM. Sorafenib is a potent inhibitor of Raf kinase but also inhibits proangiogenic RTKs including VEGFR-2, VGFRR-3, PDGFR-β, Flt-3, c-Kit, and fibroblast growth factor receptor 1. Several trials of sorafenib in high-grade glioma are underway.
Protein kinase C inhibitors
Protein kinase C (PKC) is a family of serine/threonine kinases that are involved in cell proliferation, differentiation, angiogenesis, and apoptosis. PKCs are activated by RTKs and G-protein–coupled receptors and help regulate several signaling pathways including PI3K/Akt and MAPK. Several PKC isoforms are overexpressed in glioma cell lines. Enzastaurin is an oral serine/threonine kinase inhibitor that targets the PKC and Akt pathways. In a multicenter phase 2 open-label trial, patients with recurrent GBM were randomized to either enzastaurin or lomustine. Unfortunately, enrollment was stopped after interim futility analysis due to a lack of difference in PFS between the 2 arms. Other studies of enzastaurin in combination with radiation and/or chemotherapeutic agents are in progress.
Multitargeted inhibitors and combined therapies
In an attempt to improve the effectiveness of targeted molecular therapy, there is growing interest in using multitargeted agents that inhibit several kinases (see Table 1 ) or combinations of targeted agents with other therapies (see Table 2; Table 3 ). For example, the EGFR inhibitor erlotinib has been studied in combination with mTOR inhibitors such as sirolimus and temsirolimus. Preliminary results from these erlotinib combination studies suggest only modest efficacy, and some combinations are poorly tolerated. However, other combinations of agents may be better tolerated and several trials are underway. Because antiangiogenic agents can potentially have synergistic effects with RT, two randomized multicenter trials combining bevacizumab with radiation and temozolomide are underway. In addition, several multitargeted agents are in clinical trials for GBM including vandetanib (which targets EGFR and VEGFR), sunitinib (which targets VEGFR-2, PDGFR, c-Kit and Flt-3), and tandutinib (which targets c-Kit and Flt-3).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree