Oral Cavity
Key Points
The oral cavity has numerous subsites. These include the lip, oral tongue, floor of mouth, gingiva, hard palate, buccal mucosa, retromolar trigone, and anterior faucial pillar.
Surgery is the preferred primary therapy for the vast majority of oral cancers.
Advances in free-flap reconstructive techniques over the years have substantially improved the functional outcome.
Radiation plays an important role as an adjuvant treatment when surgical pathologic features, such as close or positive surgical margins, perineural spread, lymph-vascular invasion, and/or positive nodes suggest high risk of recurrence.
Adjuvant concurrent chemoradiation is recommended in the presence of positive surgical margins or extracapsular extension of nodal disease.
Definitive radiation, preferably with interstitial brachytherapy, is an option in lieu of surgery for early-stage lesions of the lip, buccal mucosa, and mobile tongue.
The specific treatment approaches (e.g., elective nodal treatment, ipsilateral vs. bilateral irradiation, coverage of neural pathways) vary with each subsite because of the differing patterns of spread and functional repercussions.
Although retromolar trigone and anterior faucial pillar are subsites of the oral cavity, carcinomas originating from these structures are often treated in the same way as those of the oropharynx, that is, with primary radiotherapy, except for tumors invading bony structures.
LIP
Treatment Strategy
Surgery is generally preferred in medically operable patients in the following situations:
T1 lesions (up to 2 cm in diameter) that do not involve the oral commissure. Excision of such lesions is generally simple (V or W excision with primary closure or flap reconstruction), and the functional and cosmetic outcome is satisfactory.
Younger patients who will have prolonged sunlight exposure (e.g., those engaged in outdoor work).
Diffuse superficial lesion of the vermilion or the presence of severe actinic keratosis adjacent to the carcinoma. A lip shave with oral mucosa advancement closure generally yields a good control rate with satisfactory cosmetic outcome.
Radiation is an option for lesions larger than 2 cm or those involving the commissure, in which surgical resection results in microstomia or oral incontinence. Radiotherapy can be delivered by
external beam irradiation, brachytherapy, or a combination of both, depending on the location and size of the lesion.
external beam irradiation, brachytherapy, or a combination of both, depending on the location and size of the lesion.
A combination of surgery and radiotherapy is frequently required for advanced destructive lesions, that is, those invading the bone or nerve, or with nodal involvement.
Primary Radiotherapy
Target Volume
Initial Target Volume
T2 N0: primary tumor with 2-cm margins. Elective neck irradiation is not given routinely for well-differentiated carcinomas.
T3 N0: primary tumor with 2-cm margins and submental, submandibular, and subdigastric nodes.
T2 to T3 N +: primary tumor with 2-cm margins and submental, submandibular, subdigastric, mid and low jugular nodes.
A boost volume encompasses the primary lesion with 1-cm margins and, when present, involved nodes.
Setup and Field Arrangement for External Beam Irradiation
An intraoral stent containing cerrobend is used to displace the tongue posteriorly and, if feasible, shield the alveolar ridge. The patient is immobilized in a supine position. An appositional field is used to treat the primary tumor. Field borders are determined clinically by bimanual palpation. Treatment can be given with orthovoltage x-rays or electrons. Electron energy is chosen on the basis of thickness of the lesion. The upper neck nodes are treated with lateral parallel-opposed photon fields:
Anterior border: 1 cm in front of the mandibular arch.
Superior border: splitting the horizontal ramus of the mandible.
Posterior border: midvertebral body.
Inferior border: just above the arytenoids.
Moustache field for upper lip lesion: appositional electron fields (usually approximately 15-degree gantry angle) are used to treat the facial lymphatics.
Medial border: matches the lateral border of the anterior field (primary tumor).
Anterior border: extends down from oral commissure to midmandible.
Posterior border: from the upper edge of the anterior field to just above the angle of the mandible.
Inferior border: splitting the horizontal ramus of the mandible and adjoining the upper neck field.
This field is set up clinically after designing the primary tumor and upper neck portals.
Patients with palpable node(s) receive irradiation to mid and lower neck nodes through an anterior portal. The primary lesion receives boost dose through an appositional field with orthovoltage x-rays or electrons. Nodal metastases receive boost dose with appositional electrons or glancing photon fields.
Brachytherapy
Brachytherapy is usually accomplished by afterloading 192Ir-wire implants. Whenever possible, a custom-made plastic device is placed between the lip and gum to increase the distance between the radioactive sources and alveolar structures to decrease radiation exposure to normal tissues. Occasionally, cerrobend can be incorporated in the device to provide additional protection.
Dosage
Small lesions (<1.5 cm): 50 Gy in 25 fractions followed by 10 Gy in five fractions boost dose by external irradiation or 60 to 65 Gy over 5 to 7 days by implants.
Larger lesions: 50 Gy in 25 fractions followed by 16 Gy in eight fractions boost by external beam or 20- to 25-Gy boost by implants.
Elective treatment of facial (moustache area) and upper neck nodes: 50 Gy in 25 fractions.
Palpable nodes receive boost dose to a total dose of 66 to 70 Gy depending on the size.
Dose Specification
In external beam irradiation, the dose to the primary lesion is administered at Dmax for orthovoltage x-rays and at 90% for electrons. This accounts for the difference in relative biologic effectiveness between the two beam modalities.
For brachytherapy, the dose is administered at the isodose line encompassing the lesion.
Moustache fields are irradiated with 6-MeV electrons and the dose is prescribed at Dmax
Postoperative Radiotherapy
Indications for postoperative irradiation are advanced lesions (bone involvement, extensive perineural invasion), positive margins, and multiple nodes or extracapsular extension (ECE).
In general, the radiation setup is the same as that for primary radiotherapy, but should be tailored individually depending on the location of the primary lesion (upper vs. lower lip) and on the extent of nerve and lymphatic coverage.
The dose prescription is 60 Gy in 30 fractions to areas with high-risk features, 56 Gy per 28 fractions to the surgical bed, and 50 Gy in 25 fractions to electively irradiated regions.
Background Data
Table 6.1 Control of Primary Lip Lesion By Treatment Method | |||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||
FLOOR OF MOUTH, ORAL TONGUE, AND MANDIBULAR GINGIVA
Treatment Strategy
Surgical resection is generally preferred in medically operable patients. Depending on the size, depth of invasion, and grade of differentiation of the primary tumor and nodal status, surgery may include a neck dissection.
Postoperative radiotherapy is recommended in the following situations: large primary tumor (e.g., T3 or T4); close or positive surgical margins; presence of perineural spread, vascular invasion, or both; or presence of multiple positive nodes or ECE (see Case Studies 6-1 to 6-2). Patients considered at high risk, such as those having positive section, margins, or ECE, are now recommended to receive concurrent radiation with cisplatin when there is no medical contraindication.
Primary radiotherapy is generally reserved for patients who refuse surgery or those who are borderline operable.
Postoperative Radiotherapy
Target Volume
Initial Target Volume
The entire surgical bed—site of the primary tumor, dissected neck (in most cases, neck dissection is part of the surgical treatment), and dissected draining lymphatics. Elective nodal irradiation is given in conjunction with postoperative radiotherapy to the primary tumor bed in N0 patients who are not undergoing nodal dissection.
Boost Volume
In areas of original tumor and involved nodes, a second cone-down may be made to deliver additional boost dose to the region carrying the highest risk of recurrence (e.g., sites of extracapsular nodal disease or positive margins).
Setup and Field Arrangement for Conventional Radiotherapy Technique
The patient is immobilized in a supine position with a thermoplastic mask. For tumors of the oral tongue or floor of mouth that extend close to or into the tongue, an intraoral stent is used to open the mouth, depress the tongue, and protrude the lower lip. This device allows exclusion of a large area of the buccal mucosa, lower lip, and oral commissure from the radiation portals. For tumors of the floor of mouth not extending into the oral tongue, a stent that opens the mouth and elevates the tip of the tongue beyond the field is used to exclude as much of the tongue (tip, dorsum) from the radiation portal as possible. Marking of surgical scar and oral commissures before obtaining a simulation film may facilitate portal design.
Tumors of the mandibular gingiva or small tumors of the oral tongue that are well lateralized without histologic evidence of lymph node involvement can be treated ipsilaterally. This can be accomplished with a wedged pair of photon beams designed to encompass the operative bed with 1 to 2 cm surrounding margins.
The remaining tumors that require bilateral radiation are treated with parallel opposed-lateral photon fields encompassing the primary tumor bed and upper neck nodes.
Anterior border: just in front of the mandible for anterior tumors or 2 cm in front of the scar for posterior oral cavity lesions. The lower lip is shielded whenever possible.
Superior border: 1 to 1.5 cm superior to the dorsum of the tongue or the scar if part of the oral tongue can be spared. In the event of positive nodes, this border should encompass the high jugular nodes to the jugular fossa.
Posterior border: usually is dictated by the surgical scar. The level II nodes are systematically irradiated. Therefore, the
posterior border is placed behind the spinous processes or farther back to cover an extended scar.
Inferior border: just superior to the arytenoids.
Case Study 6-1
A 59-year-old man presented with a 1-month history of sore mouth. Physical examination revealed a 2.5-cm ulcerative, mobile lesion in the right anterior part of the floor of mouth. The tumor involved the frenulum of the tongue, but did not cross the midline. The mobility of the tongue was normal. A 1.5-cm mobile lymph node was palpated at the right submandibular area. Biopsy of the primary tumor showed moderately differentiated SCC. Stage: T2 N1 M0. This patient underwent a wide local excision of the primary tumor and bilateral supraomohyoid neck dissections. The defect in the floor of mouth was repaired with a split-thickness skin graft. At the time of surgery, the tumor was found to involve the sublingual gland and the deep musculature. The initial deep margin was positive, but was converted to negative by further excision of the muscle. Of the five nodes recovered from the right neck specimen, one submandibular node measuring 2 cm was involved with carcinoma without evidence of ECE. Seven lymph nodes recovered from the left side were all free of tumor. Pathologic stage: p T4 N1. The patient received postoperative radiotherapy.
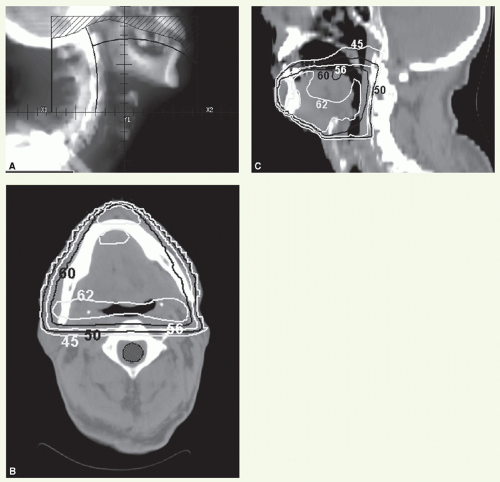
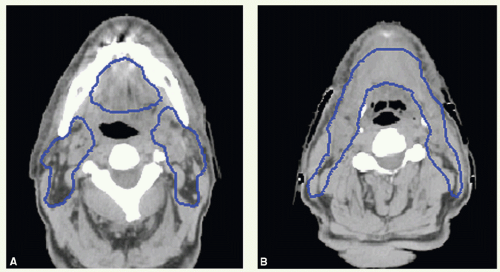
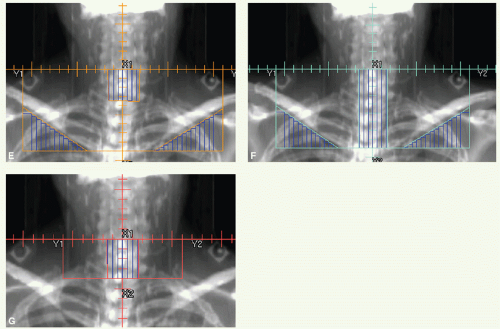
The primary site and upper neck were treated with parallel opposed-lateral photon fields (Fig. 6.1). By using a tongue depressor, the hard palate and part of the soft palate and upper gums could be excluded from the radiation field. The mid and lower neck nodes were treated with an anterior appositional photon field with a midline block to shield the larynx. This block was placed just above the surgical scar in the neck, which is marked with a wire. The primary tumor bed and uninvolved nodal areas received 56 Gy in 28 fractions. The involved nodal area was boosted to 60 Gy in 30 fractions. The boost dose to the right upper neck was delivered with an appositional electron field. He had no evidence of disease and was free of complications 2 years after treatment.
Case Study 6-2
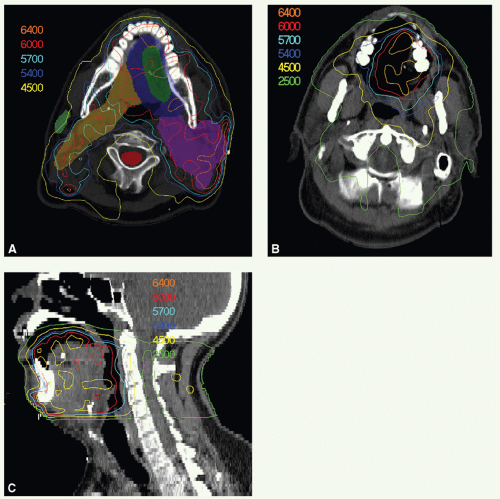
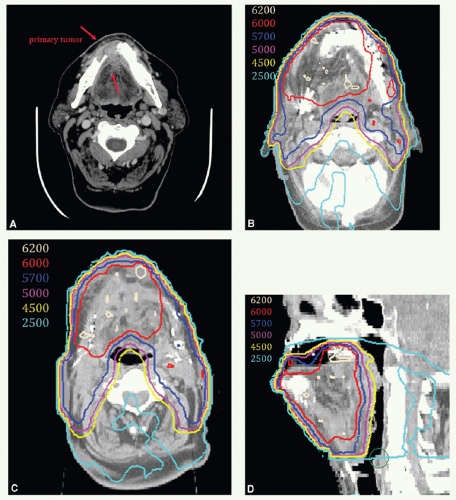
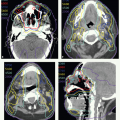
A 65-year-old man presented with ill-fitting dentures and oral pain. Physical examination revealed a 4-cm tumor on the right lateral tongue, an enlarged submandibular gland, and a 3.5-cm conglomerate nodal mass in right level II. Stage T2 N2b M0. He underwent a partial glossectomy and selective right neck dissection. Histologic examination showed an SCC of the tongue with negative section margins but presence of perineural invasion. Five of forty dissected nodes were positive, and there was ECE. He received adjunctive postoperative radiotherapy delivered with 6-MV photons for the entire treatment using an isocentric technique. A 3-mm tissue-equivalent bolus was placed over the scar.
A digitally reconstructed radiograph with the fields is shown in Figure 6.2A. An off-spinal cord reduction was made at 42 Gy and a boost cone-down at 56 Gy. The posterior cervical strips were supplemented with 9-MeV electrons, 18 Gy to the right strip and 8 Gy to the left strip. Figure 6.2B,C: show axial and sagittal isodose distributions through the tongue. The supraclavicular field had a larynx (and spinal cord safety) block. Both sides of the lower neck received 50 Gy. The right mid and lower neck received 6-Gy boost dose. These fields were further reduced off the inferior border to take the right neck at risk to 60 Gy.
Case Study 6-3
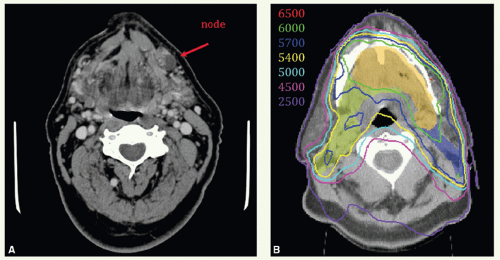
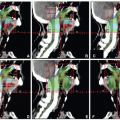
A 38-year-old man treated with a limited resection of an oral tongue SCC presented 2 years following surgery with a recurrence. He underwent a left partial glossectomy with a radial forearm graft reconstruction and left neck dissection. The tumor had 1.3-cm depth of invasion, section margins were negative (deep margin 0.6 cm) and nodes were not involved. Due to the recurrent nature and depth of invasion, he was treated with postoperative radiation using IMRT.
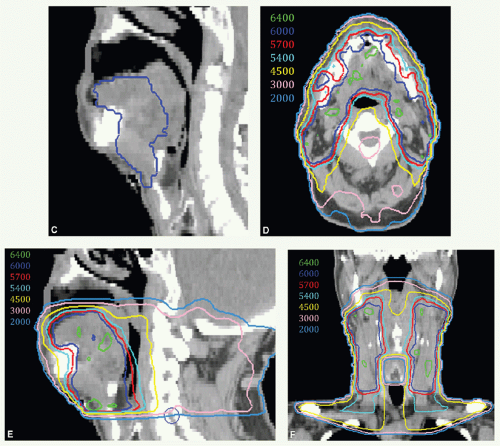
Figure 6.3A shows an axial view with contours of target volumes. The green volume represents the preoperative disease (vGTV) and blue volume the CTV to receive 60 Gy (CTVHD). The purple volume represents the remaining operative bed (including a wired scar), planned to 57 Gy (CTVID), while the orange contour represents the remaining tongue and contralateral neck nodes planned to 54 Gy (CTVED). Figure 6.3B,C show isodose distributions through a superior axial cut and a sagittal view through midplane, respectively. The patient remains well 2 years from all therapy with good speech and oral comfort.
An anterior appositional field is used to treat the mid- and lower neck nodes. Field borders are indicated in the section “General Principles.”
To deliver the boost dose to the primary tumor and upper neck nodes, the size of lateral fields is reduced to encompass the known disease locations. It is also prudent to cover the root of tongue or deep floor of mouth muscles to the insertion at the hyoid bone in the boost volume since recurrence tends to occur in these regions even for relatively superficial tumors.
To boost the upper neck without the primary site, a lateral appositional electron field is used. To deliver the boost to the mid or lower neck, a lateral appositional electron field or glancing photon fields are used.
Dose
A dose of 60 Gy in 30 fractions is administered to areas with high-risk features, that is, close or microscopically positive margins, perineural extension, vascular invasion, positive nodes, or extranodal extension. An additional boost dose of 6 Gy in three fractions may be given when indicated, such as when multiple adverse features are present or when the interval between surgery and radiation is much longer than 6 weeks.
A dose of 56 Gy in 28 fractions to the surgical bed.
A dose of 50 Gy of elective irradiation in 25 fractions to undissected regions.
Intensity-Modulated Radiation Therapy
Conformal radiotherapy can eliminate sequential portal cone-down because the dose gradient to the tumor bed and to regions at risk for harboring microscopic disease is achieved by varying the fraction size. When using intensity-modulated radiation therapy (IMRT) in the postoperative setting, treatment is given in 30 fractions as illustrated in Case Studies 6-4, 6-5, 6-6, 6-7 and 6-8. Smaller target volumes considered to be at extra-high risk, often determined in collaboration with the surgeon and based on pathologic findings, receive 64 to 66 Gy.
Case Study 6-4
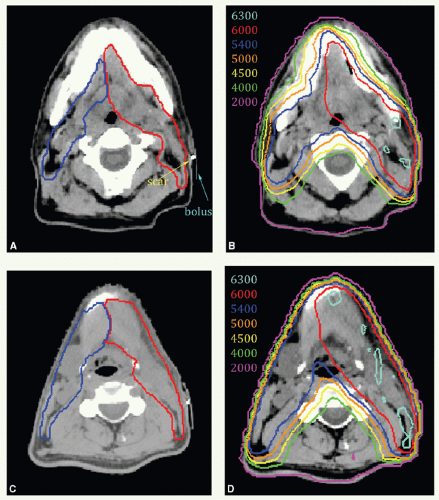
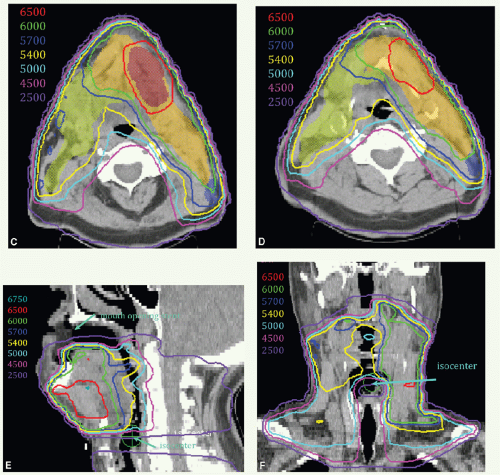
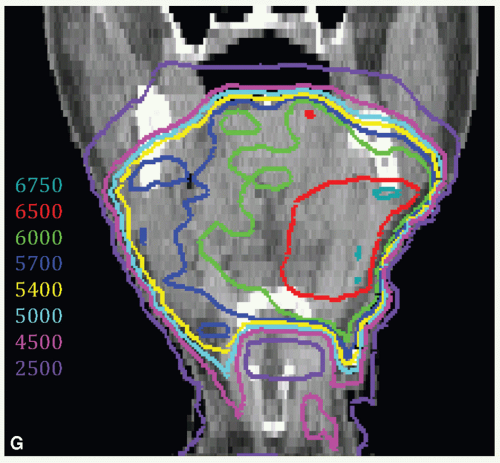
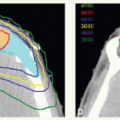
A 47-year-old man presented with biopsy-proven SCC of the left oral tongue. He underwent a partial glossectomy with selective left neck dissection. The primary tumor was 1.2 cm in size and had negative margins. The neck dissection yielded 3 positive nodes in levels II and III without ECE. He was treated with postoperative radiation. The tumor bed, consisting of the left lateral tongue and left upper neck, with margin was identified as CTVHD (60 Gy). The undissected right neck and right hemitongue was contoured as CTVED (54 Gy).
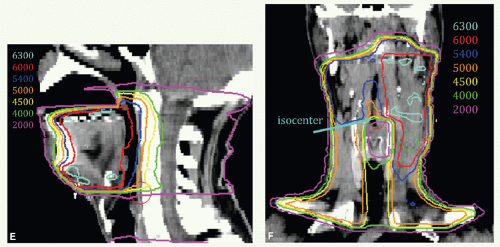
Figure 6.4 shows CTVHD (red) and CTVED (blue) paired with isodose distributions at the level of the tongue (Fig. 6.4A,B) and upper neck (Fig. 6.4C,D). Figure 6.4E,F: show isodose distributions on a sagittal view through the midtongue and a coronal view, respectively. An intraoral stent opens the mouth and separates the palate from the tongue (Fig. 6.4E). The isocenter was placed just above the thyroid notch (Fig 6.4F). IMRT was delivered to fields above the isocenter. Level III and IV nodes were treated with an anterior beam with a larynx block to 40 Gy and with a full midline block to 50 Gy. Right level III was boosted to a total of 60 Gy with glancing photon beams. The patient remains without disease 5 years later.
Case Study 6-5
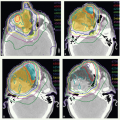
A 78-year-old man presented with an anterior oral tongue tumor and a palpable left level IB node, also detected on the staging CT scan (Fig. 6.5A). Biopsy of the primary tumor was positive for SCC. He underwent a partial glossectomy and left selective neck dissection. Histologic examination revealed a 2.5-cm carcinoma with negative margins. The neck dissection revealed only 1 positive lymph node, 2.5 cm in size consistent with the clinical findings. There was ECE. He was treated with postoperative IMRT. Concurrent chemotherapy was indicated but not given due to concern about tolerance secondary to overall performance status and age.
The tumor bed, consisting of the anterior tongue and left upper neck, with margin was identified as CTVHD (60 Gy—orange). Dissected ipsilateral level IIb was contoured
as CTVID (57 Gy—blue). The undissected right neck and an additional 0.5 to 1 cm on the tongue were identified as CTVED (54 Gy—yellow). The region of the positive node at left level IB was delineated to give a simultaneous integrated boost to a total dose of 65 Gy (red). Representative axial slices of the contours and isodose distribution from the level of the midtongue to inferior level II of the neck are shown in Figure 6.5B-D. The isocenter was placed just above the thyroid notch. IMRT was delivered to fields above the isocenter. Level III and IV nodes were treated with an anterior beam with a larynx block to 40 Gy and with a full midline block to 50 Gy. Right level III was boosted to 60 Gy with glancing photon beams. Also shown are isodose distributions through the isocenter in sagittal (Fig. 6.5E) and coronal (Fig. 6.5F) view and through the midtongue and level IB in coronal view (Fig. 6.5G). The patient remains without disease 3 years later.
as CTVID (57 Gy—blue). The undissected right neck and an additional 0.5 to 1 cm on the tongue were identified as CTVED (54 Gy—yellow). The region of the positive node at left level IB was delineated to give a simultaneous integrated boost to a total dose of 65 Gy (red). Representative axial slices of the contours and isodose distribution from the level of the midtongue to inferior level II of the neck are shown in Figure 6.5B-D. The isocenter was placed just above the thyroid notch. IMRT was delivered to fields above the isocenter. Level III and IV nodes were treated with an anterior beam with a larynx block to 40 Gy and with a full midline block to 50 Gy. Right level III was boosted to 60 Gy with glancing photon beams. Also shown are isodose distributions through the isocenter in sagittal (Fig. 6.5E) and coronal (Fig. 6.5F) view and through the midtongue and level IB in coronal view (Fig. 6.5G). The patient remains without disease 3 years later.
Case Study 6-6
A 66-year-old man presented with a 2.5-cm anterior floor of mouth tumor with extension onto the ventral tongue without palpable lymph adenopathy (T2 N0). He underwent a partial glossectomy and bilateral supraomohyoid neck dissections. Histologic examination revealed a 1.8-cm SCC with negative margins. The neck dissection specimen contained 5 positive nodes, 2 in left level III, 2 in right level I, and 1 in right level II compartments. There was no ECE. He received postoperative radiation using IMRT to the primary tumor bed and upper neck and a matching anterior portal for lower neck as described Case Study 6.7.
Since the positive nodes were scattered through the neck, and were not well identified on preoperative imaging,
bilateral level I and II nodes, the floor of mouth and anterior tongue, and the musculature that inserts onto the hyoid bone were defined as CTVED (60 Gy).
bilateral level I and II nodes, the floor of mouth and anterior tongue, and the musculature that inserts onto the hyoid bone were defined as CTVED (60 Gy).
Figure 6.6 shows target volumes on 2 axial images at the levels of the mandible (Fig. 6.6A) and upper neck (Fig. 6.6B) and sagittal image (Fig. 6.6C) along with isodose distributions on axial (Fig. 6.6D), sagittal (Fig. 6.6E), and coronal (Fig. 6.6F) views. The patient is without disease 2 years later.
Case Study 6-7
A 67-year-old man who presented with oral pain was found to have a tumor of the anterior floor of mouth that spilled over the anterior gingiva. CT scan revealed a lesion in the floor of mouth eroding the mandible (Fig. 6.7A). He underwent surgical resection, including an anterior mandibulectomy, and bilateral supraomohyoid neck dissections followed by reconstruction with a fibula free flap. Histologic examination revealed SCC, poorly differentiated, invading the bone. There was carcinoma in situ at the tongue margin.
He received postoperative IMRT to the primary tumor bed and upper neck. The tumor bed, consisting of the floor of mouth, resected right mandibular bed and anterior tongue with margin was identified as CTVHD (60 Gy). The dissected necks that did not harbor disease were contoured as CTVID (57 Gy).
Figures 6.7B-D show isodose distributions on axial images at the level of the reconstructed mandible and flap (Fig. 6.7B) and upper neck (Fig. 6.7C), and a sagittal view through midplane (Fig. 6.7D).
The IMRT beams were matched at an isocenter, placed above the thyroid notch, to an anterior portal for treating the lower nodal stations. The initial anterior beam had a larynx block and was treated to 40 Gy (Fig. 6.7E). A full midline block was added and the fields treated to 50 Gy (Fig. 6.7F). Bilateral level III, part of the operative bed, was boosted to 56 Gy with an anterior beam (Fig. 6.7G). The patient remains without disease 3 years later.
Case Study 6-8
A 49-year-old man presented to his dentist with left-sided oral pain and was found to have a lesion of the left alveolar ridge. A biopsy was positive for SCC.
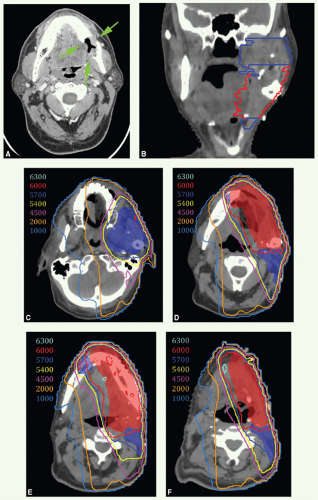
A diagnostic CT scan revealed an invasive primary tumor eroding the left mandible as shown in Figure 6.8A (green arrows). Treatment began with a wide local resection, including left mandibulectomy, and left neck dissection followed by reconstruction using an osteocutaneous free-fibular flap and skin graft. The fibula replaced the horizontal ramus whereas the soft tissue filled in the space of the resected left ascending ramus. Histologic examination revealed a 2.9-cm poorly differentiated SCC with bone invasion, but margins were negative. None of 28 nodes were positive for disease. Stage pT4 N0.
He was treated with adjuvant ipsilateral IMRT because the tumor was well lateralized and there was no nodal involvement. Figure 6.8B shows a coronal view through the right ascending ramus of the mandible with CTVHD (red) and CTVID (blue) outlined. CTVHD encompasses the preoperative tumor volume with generous margins, and CTVID covers the operative bed including generous coverage of the masticator space. The absence of the left ascending mandibular ramus can be appreciated on this coronal view. Figure 6.8C-F: show axial isodose distributions along with CTVHD (red) and CTVID (blue) at the level of the inferior maxilla and masticator space, the superior aspect of the horizontal ramus of the mandible, the fibula graft and epicenter of the original tumor, and level II nodal region, respectively. Note that CTVHD includes 1 cm of the residual mandible across midline and the upper neck to encompass soft tissues of the inferior-lateral portion of the floor of mouth musculature and tissues inferior to the angle of the mandible. The left level III and IV nodes were treated with a matching anterior beam to a dose of 50 Gy in 25 fractions. The patient had no evidence of disease 2 years after treatment.
The patient is immobilized in a supine position, with an extended thermoplastic mask covering head and shoulder. Thin-cut computed tomography (CT) images are obtained in treatment position, and target volumes are outlined for dosimetric planning.
Virtual Gross Target Volume
There is no actual GTV after completesurgicaltumorresection. However, it can be useful to formulate a virtual GTV (vGTV) to facilitate target volume definition. The vGTV is a best approximation of the tissues having high likelihood of harboring microscopic tumor reconstructed based on findings of preoperative clinical examination, imaging studies, and surgical-pathologic assessment. Bulky flaps can cause substantial distortions in the tumor bed and should, therefore, be taken into account in reconstructing the vGTV.
Clinical Target Volumes
Three CTVs are generally delineated.
CTV1 delineates volumes to receive the highest dose (therefore also referred to as CTVHD). This includes the primary and nodal vGTVs with 1-cm margins. For larger primary tumors, CTVHD often covers the entire tongue and floor of mouth.
CTV2 delineates volumes to receive an intermediate dose (therefore also referred to as CTVID). For the primary tumor bed, CTVID encompasses the remaining operative bed and/or a 0.5- to 1-cm additional margin beyond CTVHD. For the neck, it covers the dissected neck not harboring involved nodes.
CTV3 delineates volumes to receive an elective dose for subclinical disease (therefore also referred to as CTVED). In the N0 neck, nodal levels I to IV are included in CTVED When microscopic perineural invasion is present, CTVED includes the lingual nerve and/or inferior alveolar nerves to approximately the distal end of the mandibular nerve (V3) either ipsilaterally or, for tumors extending to or crossing midline, bilaterally. For extensive perineural extension (involvement of large nerve or presence of clinical signs), CTVED includes the proximal V3 up to the skull base or even the trigeminal ganglion.
The isocenter is generally placed above the arytenoids. Level III and IV nodes are preferentially treated with a matching anterior beam similar to conventional techniques. With this technique, the larynx can be shielded for the initial 40 Gy, and then a full midline block can be used up to 50 Gy. The dissected uninvolved nodal levels are boosted to 56 Gy, and an additional 4 Gy is added if these lower neck nodes harbored disease.
Some patients have extensive reconstruction with large flaps. If the bulky flaps extend at the level of the larynx or more inferiorly, matching the low neck beam to the IMRT fields may be more complicated, and it may be difficult to get the appropriate dose to the neck tissues at risk deep to the flaps. In these cases, treating all the targets with a single IMRT plan may be more effective, though additional attention should be paid to delineating the larynx and esophagus as avoidance structures for minimizing the dose to these organs.
Timing of Postoperative Radiotherapy
It is desirable to commence postoperative radiotherapy as soon as possible after healing of surgical wounds. With good communication between surgical, radiation, and dental oncologists, simulation can usually take place 3 to 4 weeks after surgery, and radiotherapy can start within a week in most patients. When delayed wound healing postpones commencement of postoperative radiation to beyond 5 to 6 weeks, we administer accelerated fractionation, such as concomitant boost, by delivering twice-a-day irradiations for 5 treatment days, either once a week or toward the end of the radiation course, to reduce the potential hazard of prolonged cumulative treatment time.
Primary Radiotherapy
Target Volume
Initial Target Volume
A well-differentiated, superficial lesion of 1 cm or less with no palpable lymphadenopathy (T1 N0): primary tumor with 2-cm margins.
Floor of mouth (mostly anterior) lesion of 1- to 4-cm maximal diameter without palpable lymphadenopathy (T1 to T2 N0): primary tumor with at least 2-cm margins and level I (submental and submandibular) and Level II nodes.
Oral tongue tumor >1 cm thick with no palpable lymphadenopathy: primary tumor with at least 2-cm margins and level I to IV nodes.
Presence of lymphadenopathy (N+) at diagnosis calls for irradiation of the entire cervical nodal basins.
A boost volume encompasses the primary tumor (1- to 2-cm margins) and involves lymph nodes.
Setup and Field Arrangement
For small T1 N0 lesions, the entire treatment is given with an intraoral cone or by implant (if the risk for anesthesia is low).
For all other stages, treatment is given with external beam irradiation by conventional technique or IMRT as described above for postoperative radiotherapy. Use of stent, insertion of a seed at the anterior border of the tumor, and marking the oral commissures before obtaining simulation images facilitates shaping of the target volumes.
The boost dose to the primary tumor is preferably delivered by interstitial implant. If the patient cannot undergo anesthesia, boost dose is given with orthovoltage x-rays through an intraoral cone when accessible; in this case, the boost is delivered before the start of the external beam
therapy while the tumor is clearly visible and palpable. In rare cases when neither implant nor intraoral cone is feasible, the boost is delivered using an external beam encompassing the tumor with 1- to 2-cm margins.
therapy while the tumor is clearly visible and palpable. In rare cases when neither implant nor intraoral cone is feasible, the boost is delivered using an external beam encompassing the tumor with 1- to 2-cm margins.
The technique for delivering the boost dose to palpable nodes depends on the strategy selected for the treatment of the primary lesion. It could be an interstitial implant, electron beams, or photons (included in the primary boost field or with separate fields, depending on the location).
Dose
Initial Target Volume
This volume receives 40 Gy in 20 fractions if this is followed by an interstitial implant, or 50 Gy in 25 fractions if the boost is delivered by intraoral cone or external beam.
Boost
The boost dose to the primary lesion delivered by an interstitial implant is 40 Gy, specified at an isodose line approximately 0.5 cm from the tumor margin; the boost dose by intraoral cone is 15 Gy in 3-Gy fractions or 20 to 25 Gy in 2.5-Gy fractions, and by external beam is 20 Gy in 10-Gy fractions for T1 lesions or 20 to 24 Gy in 2-Gy fractions for T2 to T3 lesions.
The dose to involved lymph nodes is in general 66 to 70 Gy for < 3-cm nodes, or even higher for larger nodes if neck dissection is not contemplated because of anesthesia risk.
Small (<1 cm), superficial lesions may be treated with the intraoral cone (40 Gy in 10 fractions) or by an implant only (60 Gy in approximately 6 days).
Background Data
Table 6.2 Floor of Mouth Cancer: Failure to Control the Primary Tumor By Irradiation Modalities—January 1948 Through December 1968 | ||||||
|---|---|---|---|---|---|---|
|