Treatment Strategy
Primary radiotherapy is preferred for T1 to T2 N0 to N1 carcinomas. Superficial T1 lesions without lymphadenopathy may be amenable to local excision only.
Combination of radiation with systemic therapy is the treatment of choice for T3 and selected T4 or N2 to N3 tumors. As presented in
Chapter 1, outside the protocol study setting, the three currently available treatment options established by randomized trials are radiation with concurrent cisplatin (either conventional fractionation plus three cycles of cisplatin or accelerated fractionation in 6 weeks plus two cycles of cisplatin), radiation with cetuximab (antibody against epidermal growth factor receptor), and a triple-agent induction chemotherapy regimen, referred to as TPF (docetaxel, cisplatin, and fluorouracil), followed by radiotherapy ± carboplatin. Radiation with concurrent high-dose cisplatin (100 mg/m
2, given every 3 weeks) has the longest track record and the strongest evidence-based results.
There is now a consensus that patients presenting with N2 to N3 nodal disease achieving a complete clinical and radiographic response do not require a planned neck dissection. The value of PET-CT scan obtained 10 to 12 weeks after the completion of therapy in determining the need for planned neck dissection for those with small, indeterminate residual nodal mass on CT scan is being investigated.
T4a tumors with bone invasion or extensive normal tissue destruction resulting in deformation and/or impaired functions are best treated with surgery and postoperative radiotherapy and, in the presence of extracapsular extension or positive margin, combined with concurrent cisplatin.
Primary Radiotherapy
Target Volume
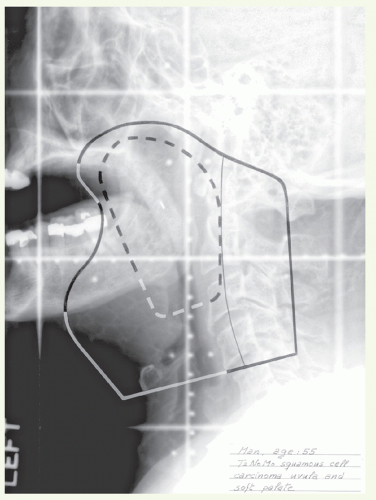
The initial target volume is primary tumor with at least 2-cm margins and bilateral neck nodes, including the retropharyngeal and level II, III, and IV nodes. The target volume also includes ipsilateral level IB in the presence of level II node(s). For lateralized tumors, the target volume includes the ipsilateral tonsillar pillars, parapharyngeal space, and lateral aspect of the pterygoid muscle.
The boost volume encompasses the primary tumor and involved node(s) with 1- to 2-cm margins.
Setup and Field Arrangement for Conventional Radiotherapy Technique
Insertion of metal seeds at the borders of the tumor, when feasible, and marking of oral commissures and palpable nodes facilitate portal shaping. The patient is immobilized in a supine position with thermoplastic mask. An extended head and shoulder mask is used for conformal radiotherapy. With conventional technique, lateral parallel-opposed photon fields are used to treat the primary tumor and upper neck nodes
(see Case Study 8-1).
Anterior border: at least 2 cm anterior to the tumor.
Superior border: at least 1.5 cm above the soft palate. If the primary tumor spreads into the tonsillar fossa, this border
is extended superiorly to encompass the medial pterygoid muscle to the pterygoid plate.
Posterior border: behind the mastoid tip when N0, behind the spinous processes when N+, or more posteriorly in the presence of large nodal mass.
Inferior border: just above the arytenoids except when the extent of nodal disease requires a lower inferior border.
A matching anterior appositional photon field is used to treat the mid and lower neck nodes when indicated. For the boost volume:
Dose
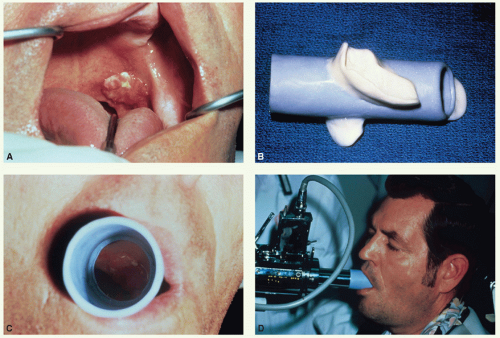
For patients with T1 N0 and superficial T2 N0 tumors: conventional fractionation delivering 50 Gy in 25 fractions to the initial target volume followed by a boost dose of 16 Gy in 8 fractions by external beam or 15 Gy in 5 to 6 fractions given by intraoral cone.
For patients with larger T2 N0 to N1 tumors or T3 to T4 tumors who do not receive systemic treatment: concomitant boost schedule to total doses of 72 Gy in 42 fractions. The initial volume receives 1.8-Gy fractions to 54 Gy in 6 weeks. The boost volume receives an additional 1.5 Gy to a dose of 18 Gy given as second daily fractions during the last 2.5 weeks of the wide-field irradiations. The spinal cord dose is limited to 45 Gy or less.
For patients with T3 to T4 or N2 to N3 tumors who receive systemic therapy: options are (1) conventional fractionation (70 Gy in 35 fractions over 7 weeks) with three cycles of concurrent cisplatin or after TPF induction chemotherapy (± carboplatin), or (2) accelerated fractionation in 6 weeks,
either by concomitant boost regimen (72 Gy in 42 fractions as described above) or in 2-Gy fractions, 6 fractions per week (1 day a week of twice-a-day irradiation), when combined with two cycles of cisplatin or weekly cetuximab.
Intensity-Modulated Radiation Therapy
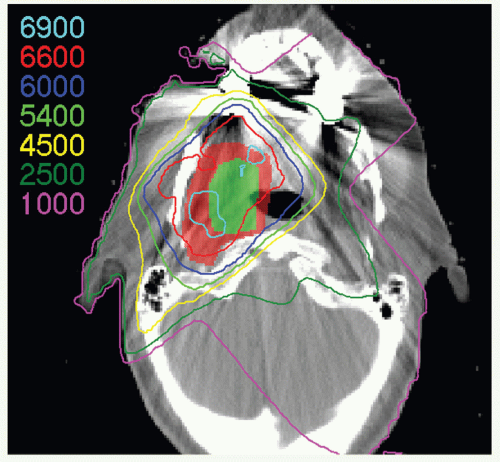
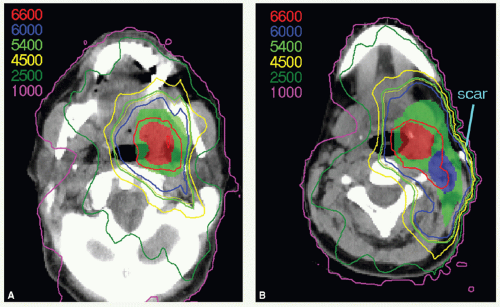
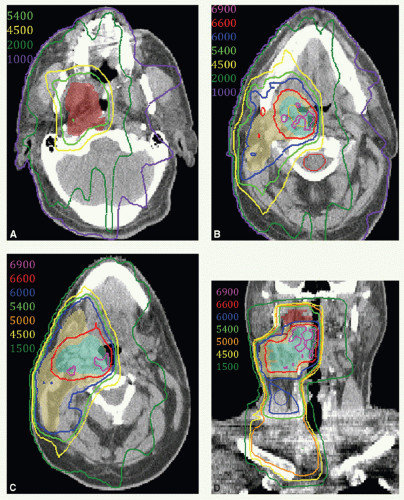
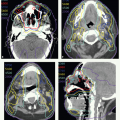
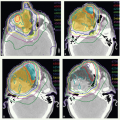
IMRT has now been widely adopted for the treatment of patients with oropharyngeal carcinomas because of its potential for exclusion of a large portion of at least one of the parotid glands from the high-dose volume, thereby reducing xerostomia without compromising the coverage of the primary tumor and draining lymphatics. The patient is immobilized in a supine position with an extended head and shoulder thermoplastic mask. Thin-cut CT scans are obtained in treatment position. The gross target volume (GTV), CTVs, and planning target volumes (PTVs) are outlined for dosimetric planning
(see Case Study 8-3).
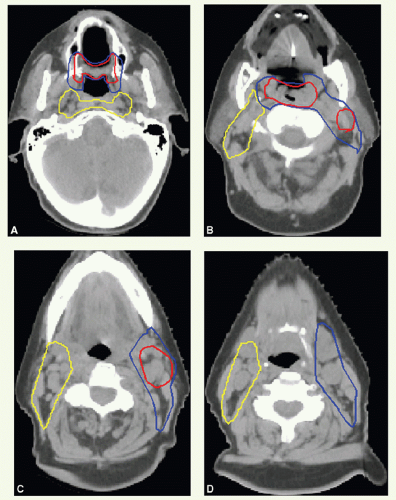
Gross Target Volume
GTV represents all areas determined from clinical examination and imaging studies to contain gross disease. It is very important to integrate the physical examination findings in treatment planning as CT scan may not detect superficial mucosal tumor extension.
Clinical Target Volumes
Three CTVs are generally delineated.
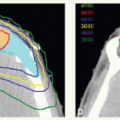
CTVHD delineates volumes to receive the highest dose, which includes the primary and nodal GTVs with 0.5- to 1-cm margins. Margins should be more generous if the tumor borders are less well defined.
CTVID delineates volumes to receive an intermediate dose, which includes the remaining soft palate and the adjacent parapharyngeal space. CTVID covers the superior aspect of the tonsillar pillars for lateral tumors and, in the presence of positive node(s), the adjoining nodal compartment(s).
CTVED delineates volumes to receive an elective dose for potential subclinical disease. In the N0 neck, CTVED includes nodal levels II to IV and retropharyngeal nodes. When level II node is involved, CTVED also includes clinically uninvolved ipsilateral level IB nodes.
Dose
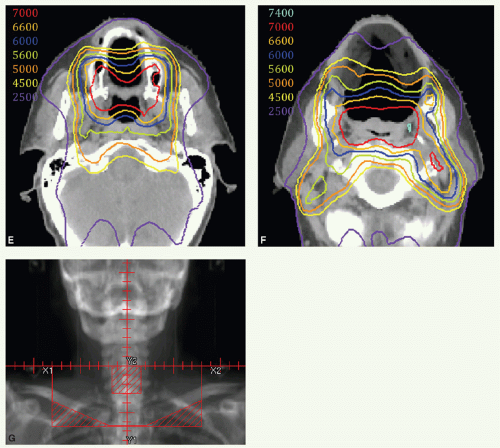
For patients with T1 N0 and superficial T2 N0 tumors: 66 Gy to CTVHD, 60 Gy to CTVID, and 54 Gy to CTVED, given in 30 fractions over 6 weeks.
For patients with larger tumors who do not receive systemic therapy: options are (1) 70 Gy to CTV
HD, 63 Gy to CTV
ID, and 56 Gy to CTV
ED given in 35 fractions over 6 weeks (1 day a week of twice-a-day irradiation), (2) 70 Gy to CTV
HD, 60 Gy to CTV
ID, and 57 Gy to CTV
ED given in 33 fractions over 6.5 weeks, or (3) a concomitant type regimen, which requires two IMRT plans (see IMRT Section of
Chapter 1).
For patients with T3 to T4 or N2 to N3 tumors who receive systemic therapy: options are (1) 70 Gy to CTVHD, 60 Gy to CTVID, and 57 Gy to CTVED given in 35 fractions over 7 weeks combined with three cycles of concurrent cisplatin or after TPF induction chemotherapy (± carboplatin), (2) 70 Gy to CTVHD, 63 Gy to CTVID, and 56 Gy to CTVED given in 35 fractions over 6 weeks (1 day a week of twice-a-day irradiation) when combined with two cycles of cisplatin or weekly cetuximab, or (3) 70 Gy to CTVHD, 60 Gy to CTVID, and 57 Gy to CTVED given in 33 fractions over 6.5 weeks combined with high-dose cisplatin.
Postoperative Radiotherapy
Adjuvant radiotherapy is indicated in occasional patients treated with upfront surgery. The principles are similar to those for the treatment of retromolar trigone or posterior oral cavity tumors as presented in detail, including illustrative cases, in
Chapter 6.
Target Volume
The initial target volume encompasses the entire surgical bed and all nodal areas of the neck. The boost volume encompasses areas of known disease location with 1- to 2-cm margins.
Setup and Field Arrangement
The general technique is the same as that described under Primary Radiotherapy.” Marking of the external surgical scar facilitates portal design. The anterior and superior field borders or CTVs are mainly determined by the local spread of the primary tumor and the extent of surgery (scar/flap). It is prudent to include 1- to 2-cm margins beyond the mucosal scar.
Background Data
Dose
A dose of 60 Gy in 30 fractions to areas with high-risk features; that is, close or microscopically positive margins, perineural extension, vascular invasion, positive nodes, or extranodal extension. An additional boost dose of 6 Gy may be given when indicated, such as when multiple adverse features are present or when the interval between surgery and radiation is much longer than 6 weeks.
A dose of 56 Gy in 28 fractions to the surgical bed.
A dose of 50 Gy in 25 fractions to undissected regions to receive elective irradiation.
Timing of Postoperative Radiotherapy
It is desirable to commence postoperative radiotherapy as soon as possible after healing of surgical wounds. With good communication between surgical, radiation, and dental oncologists, simulation can usually take place 3 to 4 weeks after surgery, and radiotherapy can start within a week in most patients. When delayed wound healing postpones commencement of postoperative radiation to beyond 5 to 6 weeks, we administer accelerated fractionation, such as concomitant boost, by delivering twice-a-day irradiations for 5 treatment days, either once a week or daily toward the end of the radiation course, to reduce the potential hazard of prolonged cumulative treatment time.