Abstract
Although breast cancer can metastasize to various sites, involvement of the ovaries is infrequent. The current study aims to report a case of ovarian cancer originating from the breast along with an in-depth analysis of the literature. A 58-year-old woman with a history of invasive lobular carcinoma underwent surgery followed by adjuvant chemo-radiotherapy. During regular follow-up, a suspicious ovarian mass was detected, later confirmed to be metastasis from the breast cancer. A review of 25 studies on Google Scholar and PubMed identified 7185 ovarian metastasis patients, with 1253 cases originating from breast cancer, accounting for approximately 17.4% of all metastatic ovarian cancers. Bilateral ovarian involvement was noted in 75% of cases, with right-side involvement in 5.7% and left-side in 19.3%. Only 13 studies documented menopausal status, showing 53.6% were premenopausal. Controversies persist in distinguishing primary ovarian cancer from metastasis using clinical signs, serum markers, and imaging. Metastasis of breast cancer to the ovaries is an uncommon event, even after utilizing various therapeutic approaches. Surgery is the treatment of choice for these cases. This case highlights the importance of long-term surveillance in breast cancer patients and emphasizes the role of surgery in managing ovarian metastases.
Background
Breast cancer stands as the most prevalent form of cancer globally, with over 2.3 million new cases each year. It is a primary cause of female mortality worldwide, with over 685,000 deaths in 2020, as reported by the GLOBOCAN database [ ]. Additionally, breast cancer frequently metastasizes to the lungs, brain, liver, and bones [ , ]. Ovarian metastasis, although less frequent in cases of breast cancer [ , ], presents unique challenges. Women diagnosed with breast cancer have 3–7 times higher chances of developing primary ovarian cancer (POC) compared to ovarian metastasis (OM) [ , , ], with the duration between breast cancer diagnosis and the development of POC tends to be shorter than in cases of breast cancer metastasizing to the ovaries [ ].
Various risk factors contribute to the development of both breast and ovarian cancers, including age, exogenous estrogen, low parity, and a family history of breast cancer due to mutations in the breast cancer genes BRCA1 and BRCA2 (6). Breast cancer spreading to the ovaries constitutes 3%-38% of all ovarian malignancies in different studies [ , , ]. The incidence rate and pattern of spread of primary malignancies significantly influence the occurrence of metastatic ovarian tumors. As the annual number of women diagnosed with breast cancer rises, so does the number of women with ovarian metastasis [ , ]. Common primary sites for ovarian metastasis are reported to be the gastrointestinal tract (39%-73%) particularly colorectal cancer and gynecological organs (10%-20%) [ ]. Although discrimination of POC from OM is difficult from a clinical perspective, imaging techniques like ultrasound can provide beneficial information, as metastatic lesions in the ovaries are smaller, have more solid components, are bilateral, and show substantial extra-ovarian spread [ , , ]. Metastatic ovarian tumors are typically chemo- and radio-resistant; however, cytoreductive surgery has been shown to have beneficial effects on symptom relief and prognosis [ , , , ]. The current study aims to report a case of a female patient with ovarian metastasis originating from breast cancer, along with an in-depth analysis of the relevant literature.
Case presentation
Patient information
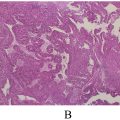
A 58-year-old female patient, with no significant family history of breast or ovarian cancer, was diagnosed with left-sided breast cancer in 2019 after presenting with a palpable breast lump and nipple retraction. A core needle biopsy confirmed invasive lobular carcinoma. Staging investigations, including contrast-enhanced computed tomography and bone scintigraphy, revealed no distant metastases at the time of diagnosis. She subsequently underwent a modified radical mastectomy with axillary clearance, with histopathological examination revealing a T3N3M0 tumor stage due to multiple positive axillary lymph nodes. Immunohistochemical studies indicated positivity for estrogen (ER) and progesterone receptors (PR), while human epidermal growth factor receptor (HER2) was negative ( Fig. 1 ). Subsequently, the patient received chemoradiotherapy and has since been maintained on hormonal therapy with an aromatase inhibitor, with regular follow-up.

Diagnostic approach
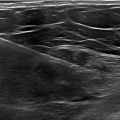
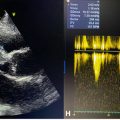
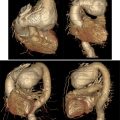
During routine follow-up, the patient underwent an abdominal ultrasound, which revealed a suspicious lesion in the left ovary, confirmed by a transvaginal ultrasound. Pelvic magnetic resonance imaging (MRI) scan revealed a 3.9 × 3.3 × 2.4 cm heterogeneously enhancing mass in the left ovary, suggestive of a malignant ovarian tumor, either metastatic or primary in origin ( Fig. 2 ). Fluorodeoxyglucose positron emission (FDG PET) scan findings were inconclusive, suggesting a benign nature of the lesion.

Laboratory investigations revealed normal levels of tumor markers, including carbohydrate antigen 125 (CA125) (12 U/mL, normal <35 U/mL), carbohydrate antigen 15-3 (CA 15-3) (18 U/mL, normal < 30 U/mL), and cancer embryonic antigen (CEA) (2.1 ng/mL, normal < 3.0 ng/mL). The lack of significant elevation in these tumor markers, typically seen in primary ovarian cancers, made differentiation between primary and metastatic disease more challenging.
Therapeutic approach
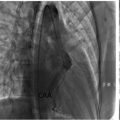
Given the indeterminate nature of the ovarian lesion and the patient’s history of hormone receptor-positive invasive lobular carcinoma, a laparoscopic hysterectomy with bilateral salpingo-oopherectomy was performed after detailed counseling and obtaining informed consent ( Fig. 3 ). Intraoperatively, no peritoneal or omental disease deposits were observed. The postoperative course was uneventful, and the patient recovered well without complications. Histopathological examination of the resected ovarian tissue revealed a left ovarian mass (measuring 4 cm) comprising metastatic lobular carcinoma of breast origin ( Fig. 4 ), confirmed through immunohistochemistry (IHC), establishing a clear link to the patient’s prior breast cancer. The Allred scoring system was used for receptor quantification, with a score of 6 for ER and PR. The score is a combination of staining intensity score (0 = none, 1 = weak, 2 = moderate, 3 = strong) and positive tumor cell percentage score (0 = none, 1 = <1%, 2 = 1-10%, 3 = 11-33%, 4 = 34-66%, 5 = >66%) ( Fig. 5 ). These findings established a definitive link to the patient’s prior breast cancer.



Follow-up and outcome
Following confirmation of ovarian metastasis, the patient was referred to the medical oncologist, who initiated fulvestrant injections and palbociclib tablets as a part of a standard endocrine-based treatment regimen. She continues to undergo regular follow-ups, with no evidence of further metastatic progression to date.
Discussion
Breast cancer represents a widespread and significant global health challenge, being both frequently diagnosed and a leading cause of death [ , , ]. Generally, the discovery of a new ovarian cyst in women with a history of breast cancer tends to be benign in nature [ , ]. Although these patients face an elevated risk of POC [ , ]. Secondary ovarian malignancies comprise 5–30% of all ovarian cancers [ , , , ]. Lee et al. reported that 13.6% of their ovarian malignancy patients had metastatic ovarian cancer, while Webb et al. found OM in 28% of the 1285 cases of malignant ovarian tumors they studied. Conversely, Yada-Hashimoto documented the rate of OM at 21.1% [ , , ].
In a literature review of metastatic ovarian cancer originating from the breast, out of 7,185 OM patients, 1,253 were diagnosed with ovarian cancer of breast origin, constituting approximately 17.4% of all metastatic ovarian cancer cases. Notably, the patient in the current case was 58 years old, higher than the reported average age of 49.2 years old in the literature ( Table 1 ).
| Author/References | Places/years | Metastatic ovarian tumors (No.) | Ovarian metastasis of breast origin (No./%) | Age (years)-average | Laterality of ovarian tumor | Menopausal state | |||
|---|---|---|---|---|---|---|---|---|---|
| Left (%) | Right (%) | Bilateral (%) | Pre- menopause (%) | Post- menopause (%) | |||||
| Tserkezoglou et al. [ ] | Greece-2006 | 36 | 9 (25% | 54 | 22% | None | 78% | None | None |
| Bigorie et al. [ ] | France-2010 | 29 | 29 (100%) | 48 | 76% | None | 24% | 62% | 38% |
| Lee et al. [ ] | Korea-2009 | 112 | 20 (1.8%) | 46,8 | None | None | 54.50% | 65.20% | 34.80% |
| Pimentel et al. [ ] | France-2016 | 28 | 28 (100%) | 52 | 36% | None | 64% | None | None |
| Yada-Hashimoto [ ] | Japan-2003 | 64 | 9 (14.1%) | 50.3 | None | None | None | None | None |
| Peters et al. [ ] | Netherlands-2017 | 2648 | 63 (2.3%) | 37 | 40.40% | None | 59.60% | None | None |
| Ayhan et al. [ ] | Turkey-2005 | 154 | 35 (22.7%) | 42.8 | None | None | None | 67% | 33% |
| Naito et al. [ ] | Korea-2012 | 1 | 1 (100%) | 54 | None | None | 100% | None | 100% |
| Alvarado/Cabrero et al. [ ] | Mexico-2013 | 150 | 20 (13%) | 51 | None | None | 57.30% | 61.30% | 38.70% |
| Bruls et al. [ ] | Netherlands-2015 | 2312 | 330 (14.3%) | 49.5 | 14.50% | 21.50% | 64% | None | None |
| Demopoulos et al. [ ] | USA-1987 | 96 | 32 (33.3%) | 54.8 | 25% | 31.30% | 43.70% | 42.70% | 57.30% |
| de Waal et al. [ ] | Netherlands-2009 | 116 | 32 (27.6%) | 49.5 | None | None | 69% | 44.00% | 39.70% |
| Kondi-Pafiti et al. [ ] | Greece-2011 | 97 | 15 (15.5%) | 55 | 22.70% | 24.70% | 52.60% | 44.30% | 55.70% |
| Moore et al. [ ] | USA-2004 | 59 | 5 (8.2%) | 55 | 20.30% | 13.60% | 66.10% | None | None |
| Fujii et al. [ ] | Japan-2006 | 1 | 1 (100%) | 34 | None | None | 100% | 100% | None |
| Durga et al. [ ] | India-2018 | 1 | 1 (100%) | 33 | None | None | 100% | 100% | None |
| Sal V et al. [ ] | Turkey-2016 | 131 | 17 (13%) | 50 | None | None | None | None | None |
| Tingler et al. [ ] | USA-2006 | N | 526 (74.7%) | 60.3 | None | None | None | None | None |
| Kim WY et al. [ ] | Korea-2010 | 158 | 3 (1.9%) | 43 | None | None | None | None | None |
| Gavriilidis et al. [ ] | Greece-2012 | 1 | 1 (100%) | 53 | None | None | 100% | None | 100% |
| Van Dam et al. [ ] | Belgium-2007 | 1 | 1 (100%) | 65 | None | None | 100% | None | 100% |
| Ozer et al. [ ] | Turkey-2012 | 68 | 5 (7.35%) | 47.3 | None | None | None | None | None |
| Turan et al. [ ] | Turkey-2006 | 75 | 5 (6.7%) | 44 | 15.40% | None | 84.60% | None | None |
| Kawakubo et al. [ ] | Japan-2010 | 1 | 1 (100%) | 50 | None | None | 100% | None | 100% |
| Gagnon et al. [ ] | Canada-1989 | 165 | 64 (38%) | 48.6 | 36% | None | 64% | None | None |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree