Overview of Recent Advances
Head and neck cancers have been the subject of intensive laboratory research and clinical investigations. Recent advances in molecular biology techniques have facilitated research addressing molecular epidemiology, genetic predisposition, genetic tumor progression models, and so on. Because of the ease of clinical assessment and a relatively low incidence of systemic spread, head and neck cancers are good models for testing the efficacy of new therapy concepts that are aimed primarily at improving local-regional disease control. Most of the clinical radiobiology research on altered fractionations and combinations of radiation with chemotherapy or emerging agents, for example, has been conducted on patients with locally advanced head and neck squamous cell carcinoma (HNSCC).
Long-term investment in cancer research has come to fruition for certain cancers. After increasing for decades, the mortality rate of most cancers in the United States has decreased since 1975.1 More recent data, for example, showed that while the incidence of all cancer has been stable in men and has increased by 0.3% annually in women between 1993 and 2002, the overall death rate has declined by an annual rate of 1.1% during this period.2 This improvement has been attributed, at least in part, to advances in cancer treatment and better dissemination of guideline-based treatment into the community.
Many advances have been achieved in the understanding of the biology, natural history, and treatment of HNSCC. A detailed depiction of recent progress is beyond the scope of this handbook—this brief introductory summary highlights a few recent findings contributing to the better understanding of the biology of the disease and to expanding treatment options for head and neck cancers.
BIOLOGY OF HEAD AND NECK SQUAMOUS CELL CARCINOMA
Key Points
Clonal genetic changes occur early in malignant cellular transformation and in the histopathologic continuum of tumor progression.
Newer molecular assay techniques, such as comparative genomic hybridization, microarray technology, and single nucleotide polymorphism analyses, have greatly increased the ability to interrogate genetic changes and thereby improve the understanding of genetic predilection (host susceptibility) and cancer biology.
The frequency of chromosome 3p/9p losses in the normal nasopharyngeal epithelium among Chinese living in endemic regions for nasopharyngeal carcinoma (NPC) was 82.6% as opposed to 20% in low-risk populations, which supports the notion of genetic predilection.
Carcinomas of the tonsil have a relatively high prevalence of transcriptionally active, integrated human papillomavirus (HPV) DNA, indicating that HPV has an active role in the carcinogenesis, mainly through the actions of E6 and E7 oncoproteins.
The incidence of HPV-related oropharyngeal carcinomas has been increasing steadily in the industrialized world since 1970s.
Genetic Alterations
The molecular tumor progression model was initially proposed by Fearon and Vogelstein.3 This model states that
tumors progress by activating oncogenes and by silencing tumor suppressor genes (TSGs), each producing a growth advantage for a clonal population of cells, and that specific genetic events usually occur in a distinct order (multistep carcinogenesis) that is not necessarily the same for each tumor. For head and neck carcinomas, Califano et al.4 described a preliminary tumor progression model using allelic loss or imbalance as a molecular marker for oncogene amplification or TSG inactivation. They identified p16 (9p21), p53 (17p), and Rb (13q) as candidate TSGs, and cyclin D1 (11q13) as a candidate protooncogene. The results of this work support the initial observations of the colorectal molecular progression model, in that clonal genetic changes occur early in the histopathologic continuum of tumor progression. About one third of histopathologically benign squamous hyperplasias already consist of a clonal population of cells with shared genetic anomalies characterizing head and neck cancer. Identification of such early events facilitates discovery of genetic alterations associated with further transformation and aggressive clinical behavior.
tumors progress by activating oncogenes and by silencing tumor suppressor genes (TSGs), each producing a growth advantage for a clonal population of cells, and that specific genetic events usually occur in a distinct order (multistep carcinogenesis) that is not necessarily the same for each tumor. For head and neck carcinomas, Califano et al.4 described a preliminary tumor progression model using allelic loss or imbalance as a molecular marker for oncogene amplification or TSG inactivation. They identified p16 (9p21), p53 (17p), and Rb (13q) as candidate TSGs, and cyclin D1 (11q13) as a candidate protooncogene. The results of this work support the initial observations of the colorectal molecular progression model, in that clonal genetic changes occur early in the histopathologic continuum of tumor progression. About one third of histopathologically benign squamous hyperplasias already consist of a clonal population of cells with shared genetic anomalies characterizing head and neck cancer. Identification of such early events facilitates discovery of genetic alterations associated with further transformation and aggressive clinical behavior.
The introduction of newer molecular assay techniques has greatly increased the ability to detect genetic changes and thereby improve the understanding of cancer biology in general. An overview by Ha et al.5 summarizes recent findings on genetic alterations in HNSCC grouped by assay techniques, such as comparative genomic hybridization, in situ hybridization, single nucleotide polymorphism, and microarray technology, and provides excellent illustrations of the complexity of HNSCC and how that complexity will require much more research to reveal the full picture. With further validation, however, this knowledge will contribute a great deal to the development of screening strategies focusing on the earlier steps of genetic alterations required to generate an invasive tumor phenotype and to the conception of early pharmacologic or genetic therapy approaches.
Lifestyle-Related Risk Factors
Tobacco and alcohol exposure have long been recognized as the dominant risk factors for HNSCC. Other risk factors include low fruit and vegetable consumption and betel quid chewing. In an overview, Petti6 estimated that, worldwide, 25% of HNSCCs are attributable to tobacco use, 7% to 19% to alcohol consumption, 10% to 15% to dietary deficiency, and, in regions of prevalence, >50% to betel quid chewing. Carcinogenicity is dose-dependent and magnified by exposures to multiple carcinogens.
Although tobacco and alcohol consumption is estimated to account for approximately three fourths of oral and pharyngeal carcinomas in the United States,7 neoplasms develop in only a small fraction of exposed individuals. This intriguing information raised the notion of the contribution of genetic susceptibility or predisposition and other cofactors (for examples of cofactors, see “Viral Etiology” section) to carcinogenesis. The potential pathways are thought to include genetic polymorphisms influencing environmental carcinogen absorption and detoxification, individual sensitivity to carcinogen-induced genotypic alterations, and so on. These ideas can now be tested more comprehensively because of recent progress in molecular biology concepts and assay methodology. For example, the ability to identify smokers at high risk for developing cancer will have important practical clinical implications in selecting individuals for more aggressive screening programs or for enrollment into intensive chemoprevention trials.
Viral Etiology
Epstein-Barr Virus
NPC has been an excellent model for studying viral etiology in human cancer. Although the association between Epstein-Barr virus (EBV) and NPC has been recognized for about four decades, major progress has been made in this field relatively recently. For example, the EBV genome was characterized (reviewed by Liebowitz8) to consist of a linear, 172-kb, double-stranded DNA having five unique sequences separated by four internal repeats and two terminal repeats. The DNA circularizes by homologous recombination at random locations within terminal repeats in the nucleus of infected cells. The length of the terminal repeat is specific for each infected cell, and this is the basis for clonality assays, which may be useful in determining the putative primary tumor in patients presenting with nodal metastasis from an unknown source. The genome encodes several families of proteins, such as early antigens, Epstein-Barr nuclear antigens (EBNAs), and latency membrane proteins (LMPs). Many of these proteins control viral behavior and affect cell proliferation regulatory mechanisms, and are thought to play a role in transformation and carcinogenesis and to influence tumor response to therapy. EBNA-1 regulates viral genome replication during cell division and was found to induce growth and dedifferentiation of an NPC cell line not infected by EBV.9 LMP-1 seems to alter growth of epithelial cells and induce well-differentiated squamous carcinomas from human epithelial cell-line transfectants and is associated with bcl-2 expression in tumors.10,11
More work has been done on the molecular genetics of NPC. Many NPCs have been found to have deletions of the short arm, or some regions of the short arm, of chromosomes 3 and 9, suggesting the possibility of the existence of TSGs in these regions.12,13 For example, studies14,15 revealed that the combined frequency for losses of chromosome 3p/9p (bearing p16 and RASSF1A) in the normal nasopharyngeal epithelium among southern Chinese in Hong Kong (a population at high risk for NPC) was 82.6% as opposed to 20% in the low-risk populations. In contrast, latent EBV infection was detected only in high-grade nasopharyngeal dysplasia or in NPC. Consequently, it was postulated that the abnormal genetic changes in chromosomes
3p and 9 predispose nasopharyngeal cells to sustain latent EBV infection, and this combination promotes a cascade of events leading to malignancy.
3p and 9 predispose nasopharyngeal cells to sustain latent EBV infection, and this combination promotes a cascade of events leading to malignancy.
Because the presence of EBV is ubiquitous, the question of why this virus is associated with NPC in Southern China and with Burkitt lymphoma in equatorial Africa but is not clearly related to other neoplasms elsewhere in the world remains puzzling. In reviewing the literature, Chang et al.16 could not detect a clear link between EBV genotype, neoplasms, and geographical factors. They suggested that the extent of EBV diversity is likely to be greater than is currently appreciated and that carefully designed studies that are conducted in wellselected populations and are sufficiently powered to provide robust epidemiologic estimates are needed to further the understanding of patterns of EBV genetic variation and their association with malignancies in different regions.
Human Papillomavirus
The causal relation between HPVs and some human neoplasms has been established, particularly for carcinoma of the uterine cervix. Nearly all cervical cancers contain integrated HPV-DNA, most commonly of high-risk types HPV-16 and HPV-18n.17 Cell culture studies clearly demonstrated that the high-risk HPVs can transform and immortalize epithelial cells from cervix, foreskin, and oral cavity.18,19,20 In contrast, HPV-6 and HPV-11, associated more often with benign lesions, do not possess this capability.21,22 Expression of the E6 and E7 open reading frames of HPV-16 or HPV-18 genome is sufficient for immortalization.23,24
The role of HPV in head and neck carcinogenesis has also attracted attention (reviewed by Herrero25). Carcinomas of the tonsil, oral tongue, and floor of mouth were found to have a relatively high prevalence of HPV-DNA.26,27,28 The evidence implicating HPVs in carcinogenesis of tonsillar carcinomas is quite strong because these tumors not only contain HPV-DNA in most of the cells but also express readily detectable levels of HPV-RNA.29 In a series of 253 patients, Gillison et al.30 detected HPV in 25% of tumors, with HPV-16 present in 90% of the positive neoplasms. The presence of HPV was most common in oropharyngeal carcinoma occurring in individuals with no history of smoking or alcohol consumption whose tumors were of a basaloid subtype without TP53 mutation. Laboratory data showing the persistence of transcriptionally active, integrated HPV-16 DNA in an oral carcinoma cell line with features indistinguishable from those of the primary tumor31 provide strong evidence that HPV has an active role in carcinogenesis.
The question of how high-risk HPV induces cell transformation has been studied mostly in cervical cancer, and the findings have been summarized in several review articles.32,33,34,35 In a nutshell, two viral oncoproteins, E6 and E7, are crucial in the transformation process. E6 binds to and inactivates the tumor suppressor protein p53, affecting many cellular functions including impairment of DNA repair after damage by other agents and suppression of the ability of cells to die by apoptosis. E7 degrades pRb, thereby releasing transcription factors such as E2F, which in turn induces the expression of other cellular proteins. E6 and E7 can also directly bind to several other host proteins, such as Bak and p21Cip1, thereby contributing to amplification of genetic instability. The expression of E6 and E7 alone does not seem to be sufficient for transforming cells, but the additional genetic alterations necessary for neoplastic conversion remain uncertain.
In reviewing data from the U.S. National Cancer Institute’s Surveillance, Epidemiology, and End Results program registries from 1973 to 2004, Chaturvedi et al.36 noted a change in the demographics of oral squamous cell carcinoma (OSCC), that is, carcinomas arising from the mucosa of the oral cavity and oropharynx, in the United States. The incidence of HPV-related OSCC increased significantly from 1973 to 2004, particularly among white men and at younger ages. In contrast, the incidence of HPV-unrelated OSCC was stable up to 1982 and then declined significantly from 1983 to 2004. The age at diagnosis declined from 1973 to 2004 for HPV-related OSCCs (0.5-year decrease per decade; P < 0.001) but increased for HPV-unrelated OSCCs (0.7-year increase per decade; P < 0.001). Similar trends have also been reported from other Western countries, such as Sweden37 and Norway.38
An increasingly large body of data shows that the prognosis for patients with HPV-related oropharyngeal carcinomas (OPSCCs) is consistently better than for those with HPV-unrelated OPSCCs after treatment with surgery,39 radiotherapy,40,41 induction chemotherapy followed by chemoradiation,42 and concurrent radiation plus cisplatin.43 Based on this strong evidence, several clinical trials have been undertaken or are being designed to test several potentially less toxic regimens. However, until such trials yield conclusive results, head and neck oncologists should not change the current treatment policies for patients with HPV-positive OPSCCs.
BIOMARKERS
Key Points
Three strong prognostic biomarkers have emerged for HNSCC. The absence of circulating EBV DNA titer, presence of HPV in cancer cells, and low tumor EGFR expression are associated with better outcome after current standard therapies for patients with nasopharyngeal cancer, oropharyngeal carcinoma, and HNSCC not associated with EBV or HPV, respectively.
Patients with NPC and persistent circulating EBV DNA after completion of radiotherapy with concurrent cisplatin have a high distant relapse
rate and are thus suitable candidates for addressing intensification of systemic therapy.
With current standard therapies, patients with HPV-associated OPSCCs have much better local-regional control and overall survival rates than those with HPV-unrelated OPSCCs.
HPV-associated OPSCC is now considered a distinct cancer entity, and protocols focusing on reducing long-term morbidity are being designed for such patients.
The search for biomarkers that can predict the likelihood that a certain cancer subset will respond to a given therapy (predictive marker) has not yielded promising leads.
High-EGFR-expressing HNSCCs are more proficient in repairing radiation-induced DNA injury and hence recur more frequently after radiotherapy, but whether inhibitors of EGFR can preferentially enhance the radiation response of these tumors has not been resolved.
Because the cost of cancer treatment has been increasing steeply with only modest improvements in efficacy, the identification, standardization, and validation of predictive biomarkers are crucial for rational selection of specific therapies for a given subset of patients to improve outcome, reduce overall toxicity, and contain cost.
Although mortality rates from cancer have gradually declined in the United States over the past 10 years,44 the cost of cancer therapy has increased drastically during that time (American Cancer Society report on Cancer Facts & Figures 2009). This increase in cost results from progressive intensification of therapies, such as the addition of chemotherapy to radiation or to surgery plus radiation, the emergence of expensive novel agents, and the lack of validated markers to guide rational patient selection for available therapies. Consequently, expensive and complex combined therapy regimens have often been prescribed to large groups of patients that benefit only a small subset of those patients, and often at the cost of increased acute and long-term morbidity. Therefore, identification and validation of biomarkers to guide the rational selection of specific therapy for a given subset of patients have become critical for improving the outcome, reducing the toxicity burden, and containing the costs of cancer treatment.
Progress in searching for useful markers for early detection of tumor, estimation of tumor burden, prediction of response to therapy, and monitoring disease progression has been slow. A prototypical marker is prostate-specific antigen, which proved to be quite useful for prostatic cancer screening, prognostic grouping, and monitoring of response to therapy. Unfortunately, equivalent markers have yet to be identified for most other solid tumors. However, recent studies in head and neck carcinomas have generated some optimism, as discussed in the sections that follow.
Prognostic and Predictive Biomarkers
The distinction between prognostic and predictive biomarkers has not been widely appreciated. Therefore, until recently, these terms have been used rather loosely and interchangeably. Figure 1.1 illustrates the concept and definition for different classes of markers. The rates and extent of separation among the curves will vary with the disease type and stage and the efficacy of therapy, but the general principles and the relative ranking are applicable. In panel A, marker X represents an aggressive tumor feature, the presence of which is associated with poorer survival rate after both treatment (Rx) regimens 1 and 2, though Rx 2 is more effective than Rx 1. Marker Y (panel B), on the other hand, exemplifies a predictive marker for response to Rx 2. Hence, its presence is associated with better survival after Rx 2 (solid brown curve). Panel C illustrates that some markers could
have both prognostic and predictive values. The absence of marker Z is associated with better prognosis (solid and dotted black curves vs. dotted purple curve). However, since this marker also predicts response to Rx 2, its presence is associated with a better outcome after Rx 2 (solid purple curve) relative to Z+ after Rx 1 (dotted purple curve) and Z- after Rx 2 (solid black curve).
have both prognostic and predictive values. The absence of marker Z is associated with better prognosis (solid and dotted black curves vs. dotted purple curve). However, since this marker also predicts response to Rx 2, its presence is associated with a better outcome after Rx 2 (solid purple curve) relative to Z+ after Rx 1 (dotted purple curve) and Z- after Rx 2 (solid black curve).
Figure 1.1 shows that carefully designed clinical trials incorporating patient stratification according to biomarkers and randomizing patients to received distinct therapy modalities are needed to yield a conclusive answer as to whether a marker is prognostic, predictive, both, or neither.
Three potent prognostic biomarkers for HNSCC have emerged in recent years. Two of these biomarkers are related to virus-associated head and neck carcinomas: circulating EBV titer for NPC and the presence of the HPV genome or its surrogate marker, p16, for OPSCC. The third biomarker, epidermal growth factor receptor (EGFR), seems to be more applicable for other HNSCCs.
Circulating EBV DNA Titers in Nasopharyngeal Carcinoma
The association between EBV and NPC was summarized in a previous section. Lo et al.45 have developed a real-time quantitative polymerase chain reaction assay for measuring circulating levels of tumor-derived EBV DNA in the serum or plasma of patients with NPC. They found in a longitudinal follow-up of 17 patients that elevations in serum EBV DNA titer could be detected as early as 6 months before clinical manifestation of recurrence, whereas the titer stayed low or undetectable in patients who remained in remission. A subsequent study of patients treated with radiation, with or without chemotherapy, at the same center46 showed that having a pretreatment EBV DNA titer exceeding 4,000 copies per mL was associated with a 2.5-fold higher risk of NPC recurrence. More interestingly, having a high posttreatment EBV DNA titer was found to be an even stronger marker for poor overall outcome, that is, a 11.9-fold increase in recurrence rate. Conversely, having a posttreatment titer of <500 copies per mL was associated with favorable overall survival and relapse-free survival rates (Fig. 1.2) and also correlated with low relapse rate. Similar results were reported by Lin et al.47 in a series of patients treated with weekly neoadjuvant chemotherapy (cisplatin alternating with fluorouracil for a total of 10 doses) followed by radiotherapy.
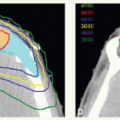
The combination of intensity-modulated radiotherapy (IMRT), as discussed in the section “High-Precision Radiotherapy” below, with concurrent cisplatin has yielded local-regional control rates of around 90% even among patients presenting with locally advanced NPC. Consequently, distant metastasis has become the main pattern of relapse for this neoplasm. Therefore, plans are underway to select high-risk populations, consisting of those with persistent circulating EBV DNA after receiving the combination of IMRT with cisplatin, for testing the efficacy of combinations of novel agents with conventional chemotherapy for eliminating occult metastatic disease.
Human Papillomavirus and p16 in Oropharyngeal Carcinomas
As noted earlier in this chapter, the incidence of HPV-associated OPSCC is increasing, particularly in the Western world, and several retrospective case series have shown that patients with HPV-positive OPSCC treated with contemporary single or combined therapy modalities have a better prognosis than do patients with HPV-negative OPSCC. However, owing to small sample sizes, other favorable prognostic factors associated with tumor HPV status (e.g., earlier tumor stage, young age) cannot be excluded as potential explanations for the observed
difference in survival. Therefore, a thorough correlative study was undertaken to quantify the magnitude of impact of tumor HPV status on tumor outcome in patients enrolled in a large phase III trial of the Radiation Therapy Oncology Group (RTOG 0129) treated with a combination of radiation with concurrent cisplatin.43 Patients with locally advanced HNSCC were stratified according to tumor site (larynx vs. other), nodal status (N0 vs. N1-N2b vs. N2c-N3), and Zubrod performance status (0 vs. 1) and assigned to receive either accelerated fractionation with a concomitant boost (72 Gy in 42 fractions over 6 weeks) or standard fractionation (70 Gy in 35 fractions over 7 weeks) regimens. Chemotherapy consisted of intravenous cisplatin at a dose of 100 mg/m2 on days 1 and 22 in the accelerated fractionation group or on days 1, 22, and 43 in the standard-fractionation group.
difference in survival. Therefore, a thorough correlative study was undertaken to quantify the magnitude of impact of tumor HPV status on tumor outcome in patients enrolled in a large phase III trial of the Radiation Therapy Oncology Group (RTOG 0129) treated with a combination of radiation with concurrent cisplatin.43 Patients with locally advanced HNSCC were stratified according to tumor site (larynx vs. other), nodal status (N0 vs. N1-N2b vs. N2c-N3), and Zubrod performance status (0 vs. 1) and assigned to receive either accelerated fractionation with a concomitant boost (72 Gy in 42 fractions over 6 weeks) or standard fractionation (70 Gy in 35 fractions over 7 weeks) regimens. Chemotherapy consisted of intravenous cisplatin at a dose of 100 mg/m2 on days 1 and 22 in the accelerated fractionation group or on days 1, 22, and 43 in the standard-fractionation group.
Of the 743 patients enrolled, 60% had OPSCC. Pretreatment biopsy specimens from the patients with OPSCC were evaluated for HPV-16 DNA by using in situ hybridization, and HPV-16-negative tumors were further assayed for 12 additional oncogenic HPV types (types 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68). Tumor expression of the cyclin-dependent kinase inhibitor p16, induced as a consequence of pRb inactivation by viral oncoprotein E7,48 was evaluated by immunohistochemical staining with a mouse monoclonal antibody. Strong agreement between tumor HPV status as determined by in situ hybridization and p16 expression was observed, but some discrepancies were noted as well. Overall, 64% of OPSCCs were found to be positive for HPV-DNA and 68% were positive for p16. Figure 1.3 shows the differences in overall survival according to HPV or p16 status. Because the sensitivity for detecting non-HPV-16 types, expected to account for 5% to 10% of HPV-positive OPSCC, was not well known at the time of the study, misclassification of HPV-positive tumors as HPV-negative tumors likely explains the slightly larger reduction in risk of death when p16 expression was used in the analysis. A strength of the p16 assay is that it is not HPV-type specific and is therefore an excellent surrogate for tumor HPV status. Analysis of patterns of failure among these groups showed that the local-regional failure rate at 3 years was significantly lower for patients with HPV-positive OPSCC (13.6% vs. 35.1%, P < 0.001) but rates for distant metastasis were not (8.7% vs. 14.6%, P = 0.23). Also, the cumulative incidence of second primary tumors (SPTs) was significantly lower for patients with HPV-positive OPSCC (3-year rates 5.9% vs. 14.6%, P = 0.02), largely because of lower rates of smokingrelated cancers in that group.
Tobacco smoking was also found to be independently associated with overall survival and progression-free survival, both in patients with OPSCC and in the entire study population. Risk of death, for example, increased significantly by 1% per each pack-year increase in tobacco use, and magnitudes of effect were similar for patients with HPV-positive OPSCCs (hazard ratio [HR] 1.01, 95% confidence interval [CI] 1.00 to 1.02) and HPV-negative OPSCCs (HR 1.01, 95% CI 1.00 to 1.03).
The RTOG 0129 study also showed that HPV-associated OPSCCs were more common among people who had never smoked or had sporadically smoked and were also significantly associated with several favorable prognostic factors, including younger age, white race, better performance status, absence of anemia, and smaller primary tumors. In multivariate analysis, age, race, performance status, tumor classification, nodal classification, and tobacco pack-years were also significant determinants of overall survival. When the unadjusted hazard ratios (HR 0.38, 95% CI 0.26 to 0.55) were compared with the adjusted hazard ratios for HPV (HR 0.42,
95% CI 0.27 to 0.66), factors other than HPV were estimated to account for about 9% of the difference in overall survival between patients with HPV-positive and HPV-negative OPSCCs (Fig. 1.3A).
95% CI 0.27 to 0.66), factors other than HPV were estimated to account for about 9% of the difference in overall survival between patients with HPV-positive and HPV-negative OPSCCs (Fig. 1.3A).
Recursive partitioning analysis indicated that tumor HPV status was the major determinant of overall survival, followed by tobacco smoking (≤10 vs. >10 pack-years) and then nodal category (N0-2a vs. N2b-3) for patients with HPV-positive OPSCCs and primary tumor category (T2-3 vs. T4) for patients with HPV-negative OPSCCs (Fig. 1.4A). Recursive partitioning led to the classification of patients with OPSCC into three risk groups: low risk (reference group, with a 3-year overall survival rate of 93%), intermediate risk (HR 3.54, 95% CI 1.91 to 6.57; 3-year overall survival rate of 70.8%), and high risk (HR 7.16, 95% CI 3.97 to 12.93; 3-year overall survival rate of 46.2%) of death (Fig. 1.4B). Patients with HPV-positive OPSCC were generally at low risk except for those who smoked or had N2b-3 nodes, which put them at intermediate risk. Patients with HPV-negative OPSCC were generally at high risk, but those having no history of tobacco use and having T2-3 tumors were at intermediate risk. Based on these findings, several trials are being designed that focus specifically on patients with HPV-related OPSCC.
Tumor Expression of Epidermal Growth Factor Receptor
EGFR is composed of four extracellular domains (I to IV), including the ligand-binding regions (domains I and III), a hydrophobic transmembrane domain, a juxtamembrane domain, an intracellular protein tyrosine-kinase domain containing the ATP binding pockets, and a regulatory carboxyl terminal domain. It is monomeric in the absence of ligands. Binding of a ligand to the extracellular domains I and III alters the spatial configuration of these domains, creating an extended and stabilized conformation that promotes homodimerization and heterodimerization49 and activates signal transduction.
The EGFR signaling pathway has evoked considerable attention as a potential biomarker for radiation response. EGFR is overexpressed in many neoplasms, for example, in 80% to 100% of HNSCCs, and perturbation of EGFR signaling is regarded as a major cause of malignant transformation and progression.50,51 An extensive correlative biomarker analysis using tumor biopsy specimens from patients with locally advanced HNSCC enrolled in a phase III trial of conventionally fractionated radiation (70 Gy in 2-Gy fractions, five times a week) showed no correlation between EGFR expression and T or N classification, American Joint Committee on Cancer (AJCC) disease stage grouping, and recursive partitioning analysis classes52 (r: -0.07 to +0.17).
As shown in Figure 1.5, EGFR overexpression, defined in terms of some level above the median mean optical density or a staining index measured using an image-analysis-based immunohistochemical assay, was found to be a strong and independent marker for higher local-regional relapse rate (68% vs. 50% at 5 years, P = 0.0031) and inferior overall survival rate (20% vs. 40%, P = 0.006) but not for the incidence of metastasis.53 This finding was supported by those from another recent study using automated quantitative assessment of EGFR expression in a tissue microarray from a cohort of patients with oropharyngeal cancer treated with radiation alone, postoperative radiation, or chemoradiation.54 The investigators revealed a strong correlation between EGFR expression, defined as above median level, and worse local recurrence rate (58% vs. 17%, P < 0.01) and worse disease-free survival rate (19% vs. 43%, P = 0.0016).
Two European studies, also conducted using specimens from patients with HNSCC enrolled in major radiation
fractionation trials, showed that high EGFR expression was predictive for increased tumor response in patients treated with accelerated radiation, which suggests that EGFR is functionally related to accelerated tumor cell repopulation during fractionated radiation.55,56 In one of these studies, the HR for local-regional recurrence after conventional versus hyperfractionated and accelerated radiation was estimated to be 1.8 in the group with an EGFR index (i.e., the proportion of EGFR-positive tumor cells) above the median value (2P = 0.010, 95% CI 1.14 to 2.8). These data indicate that EGFR inhibitors could be especially beneficial in combination with hyperfractionated and accelerated radiation regimens.
fractionation trials, showed that high EGFR expression was predictive for increased tumor response in patients treated with accelerated radiation, which suggests that EGFR is functionally related to accelerated tumor cell repopulation during fractionated radiation.55,56 In one of these studies, the HR for local-regional recurrence after conventional versus hyperfractionated and accelerated radiation was estimated to be 1.8 in the group with an EGFR index (i.e., the proportion of EGFR-positive tumor cells) above the median value (2P = 0.010, 95% CI 1.14 to 2.8). These data indicate that EGFR inhibitors could be especially beneficial in combination with hyperfractionated and accelerated radiation regimens.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree