Abstract
We retrospectively reviewed the clinical presentations, diagnostic evaluations, imaging findings, and treatment outcomes of 3 patients diagnosed with pancreatic tuberculosis. All 3 patients presented with nonspecific symptoms such as epigastric pain and weight loss. Imaging showed pancreatic masses suggestive of pancreatic cancer. EUS with FNA and subsequent histopathology confirmed tuberculosis through the presence of granulomas with caseous necrosis. All patients received anti-tuberculosis therapy, leading to favorable outcomes, including pain resolution and normalization of follow-up imaging.
Introduction
Infection with Mycobacterium tuberculosis (MTB) remains one of the leading causes of mortality worldwide and continues to represent a significant public health issue, particularly in low- and middle-income countries [ ]. Currently, tuberculosis is the 13th leading cause of death worldwide and the second leading cause of death from infectious diseases, after COVID-19. Patients infected with the human immunodeficiency virus (HIV) have an 18-fold increased risk of developing active tuberculosis. Furthermore, alcohol consumption and smoking increase the risk of tuberculosis by 3.3 and 1.6 times, respectively [ ].
Abdominal tuberculosis accounts for 11%-16% of MTB cases, with the ileocecal region often being more affected than solid organs such as the kidney, spleen, and liver, and, in rare cases, the pancreas [ ]. Pancreatic tuberculosis is extremely uncommon, even in areas with high tuberculosis prevalence, with reported incidences of less than 4.7% (14/297 cases) and 2% (11/526 cases) in other studies [ ].
Diagnosing pancreatic tuberculosis is difficult due to its low incidence and nonspecific symptoms, which frequently resemble those of pancreatic cancer. Timely and accurate diagnosis is essential for initiating appropriate treatment. This not only facilitates effective pharmacological interventions in most cases but also helps prevent unnecessary and costly surgical procedures [ ]. Herein, we present a case series of 3 patients diagnosed with pancreatic TB in our Gastroenterology department.
Case 1
A 44-year-old woman, with no significant past medical history or known exposure to MTB, presented with complaints of atypical epigastric pain lasting for 2 months. The patient had previously undergone cholecystectomy for cholelithiasis several years prior, but there was no history of jaundice, fever, or unexplained weight loss. Her family history was noncontributory, with no known hereditary conditions or history of tuberculosis.
On clinical examination, there were no palpable abdominal masses, and no signs of peripheral lymphadenopathy. The patient’s vital signs were stable, and the physical examination revealed no remarkable findings.
Routine laboratory tests were conducted, which revealed no significant abnormalities. The complete blood count (CBC) showed a white blood cell count (WBC) of 5900/mm 3 , with a lymphocyte count of 1300/mm 3 . Hemoglobin (Hb) was 12 g/dL, and the serum creatinine level was within the normal range at 6 mg/L. Liver function tests, including aspartate aminotransferase (AST) and alanine aminotransferase (ALT), were normal. Additionally, the total bilirubin and gamma-glutamyltransferase (GGT) levels were within normal limits. C-reactive protein (CRP) was normal at 2 mg/L, and the albumin level was 42 g/L. Carbohydrate antigen 19-9 (CA 19-9) was also normal (<2 IU/mL), as was the angiotensin-converting enzyme (ACE) level at 1 ng/mL. Sputum smears for acid-fast bacilli (AFB) were negative; however, the QuantiFERON test returned positive, raising the suspicion of tuberculosis as a potential underlying cause ( Table 1 ).
| Case 1 | Case 2 | Case 3 | Normal range | |
|---|---|---|---|---|
| Age | 44 | 32 | 49 | |
| Sexe | F | F | F | |
| Lipasemia | 8 | 39 | 40 | 8-78 UI/L |
| Bilirubin | 6 | 4 | 4 | 2-12 mg/L |
| AST | 13 | 19 | 69 | 5-34 UI/L |
| ALT | 16 | 10 | 30 | 0-55 UI/L |
| CRP | 8 | 19 | 240 | 0-5 mg/L |
| WBC | 5900 | 10,600 | 4600 | 4000-10,000/mm 3 |
| Lymphocyte | 1300 | 2500 | 3900 | 1000-4500/mm 3 |
| HB | 12 | 12 | 7.7 | M 13-18 g/dL |
| F 12-16 g/dl | ||||
| Platelet count | 223,000 | 333,000 | 237,000 | 150,000-400,000/µL |
| Creatinine | 6 | 7 | 6 | 7-11 mg/L |
| Urea | 0.19 | 0.25 | 0.26 | 0.15-0.45 g/L |
| Albumin | 40 | 40 | 20 | 35-50 g/L |
| CA 19-9 | <2 | <2 | 6 | |
| HIV | Negative | Negative | Negative | |
| MPD dilation | No | No | No | |
| Diagnosis | Surgery | EUS + FNA | Sputum (GeneXpert) |
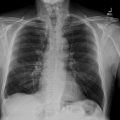
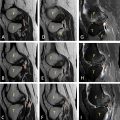
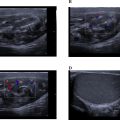
A series of imaging studies were performed to further evaluate the patient’s condition. A chest, abdominal, and pelvic CT scan revealed a well-defined, heterogeneous mass in the head of the pancreas, measuring 23 × 30 mm. There was no evidence of vascular involvement, no ascites, and no pulmonary lesions ( Fig. 1 ). Subsequently, an abdominal MRI was performed to gather further information. The MRI revealed a mass in the head of the pancreas measuring 46 × 39 mm, well-circumscribed, with specific characteristics on different imaging sequences:
- •
T1 sequence: The lesion appeared hypo-intense, with mild internal heterogeneity, suggesting a solid component and a central area of higher density. There was no marked contrast enhancement at this level.
- •
T2 sequence: The lesion appeared hyper-intense, with areas of higher signal intensity that may indicate necrosis or granulomatous inflammation.

Additionally, the MRI showed no dilation of the bile ducts or the main pancreatic duct (MPD), and no distant metastases were identified. The heterogeneous characteristics of the lesion were more apparent in the T2 sequence.
Endoscopic ultrasound (EUS) showed a 20 mm heterogeneous mass in the head of the pancreas, without dilation of the MPD. The mass was adjacent to the portal vein (PV), and there was a 30 mm lymph node at the hepatic hilum.
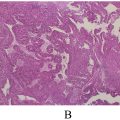
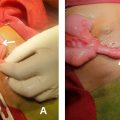
The imaging studies indicated a significant pancreatic mass that raised a strong suspicion of resectable pancreatic cancer. This urgency led to the decision to proceed with surgery without performing a cytological aspiration. The patient underwent an extended cephalic duodenopancreatectomy involving the body of the pancreas, along with retroportal and celiac trunk lymph node dissection. Histopathology showed chronic granulomatous pancreatitis, marked by epithelioid and giant cells. Granulomatous lymphadenitis was also detected, with similar findings of epithelioid and giant cells and punctate eosinophilic necrosis.
The patient was initiated on anti-tuberculosis treatment consisting of a 2-month regimen including rifampicin, isoniazid, pyrazinamide, and ethambutol, followed by 4 months of rifampicin and isoniazid alone (2RHZE/4RH).
The treatment resulted in favorable outcomes, with significant improvement in the patient’s symptoms and overall health. Regular follow-up imaging showed no recurrence of the mass or any new lesions, indicating successful resolution of the disease. The patient reported relief from her initial epigastric pain and maintained stable vital signs, with no complications arising from the surgical intervention or the anti-tuberculosis therapy.
Case 2
A 32-year-old female patient, without any notable medical history, she was previously healthy, with no known chronic conditions, and had no history of gastrointestinal disorders, pancreatitis, or any previous surgeries. The patient had no prior history of smoking, alcohol use, or substance abuse, and she was not on any regular medications. Regarding her family history, there was no known history of pancreatic diseases, tuberculosis, or any hereditary gastrointestinal disorders. She presented with atypical epigastric pain associated with weight loss. Clinical examination was unremarkable. Laboratory tests, including liver function tests, and tumor markers, were all within normal limits, however, WBC were elevated (10,600/mm 3 ) as well as a CRP level of 19 mg/l, she had a slightly higher lipase level (39 UI/L), which did not suggest acute pancreatitis. However, The patient’s liver enzymes, including AST and ALT, GGT and total bilirubine were within normal limits ( Table 1 ).
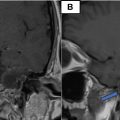
The scan revealed a 50 mm mass located in the head of the pancreas. The mass exhibited invasion into the portal trunk, celiac trunk, and common hepatic artery, which raised concern for a locally advanced pancreatic process. The tumor was heterogeneous in appearance, with both solid and necrotic components. In addition to the mass itself, the CT scan also revealed coeliac lymphadenopathy, indicative of possible lymphatic spread or reactive lymph node enlargement due to the inflammatory process. However, no signs of distant metastasis were observed, as the lungs and liver. There were no signs of peripancreatic fluid collection or ascites, which could be seen in advanced malignancy or pancreatic abscesses ( Fig. 2 ).