Paranasal Sinuses
Key Points
The paranasal sinuses include the maxillary, ethmoid, sphenoid, and frontal sinuses.
Cancers of the paranasal sinuses are uncommon with the majority originating in the maxillary sinus.
Carcinomas arising from the sinuses have a wide range of histologic types. Squamous cell cancer is most common but neoplasms arising from minor salivary glands, and respiratory and olfactory cells can also develop in sinuses.
Most sinus cancers are treated with surgery. Radiation with or without chemotherapy is often recommended postoperatively.
Rare neoplasms, including undifferentiated carcinomas, can be addressed with definitive radiation in combination with chemotherapy.
The proximity of the eyes, optic pathways, and brain makes treatment of these cancers quite challenging because of the complexity of target volumes. Intensity-modulated radiation therapy (IMRT) can circumvent some of these challenges.
MAXILLARY SINUS
Treatment Strategy
Surgery alone is the preferred treatment of T1 tumors (uncommon). Postoperative radiotherapy is only indicated when the margin is close or positive. Surgery plus postoperative radiotherapy is the standard therapy for T2 to T4 tumors.
Patients with larger T3 and T4 tumors are occasionally selected for treatment with systemic therapy in an attempt to reduce the need for orbital exenteration. The response to chemotherapy determines the type of local-regional treatment. For complete or near-complete response, the treatment is radiotherapy with concurrent chemotherapy (uncommon), and for less than near-complete response, the treatment is surgery plus postoperative radiotherapy, with concurrent chemotherapy in case of the presence of positive margins or nodal disease with extracapsular extension.
Postoperative Radiotherapy
Target Volume
The initial target volume encompasses the entire surgical bed (see Case Studies 12-1 and 12-2), ipsilateral levels IB and II (submandibular and subdigastric) nodes for patients with squamous cell or undifferentiated carcinomas with no clinical evidence of nodal involvement at diagnosis, or whole ipsilateral or bilateral neck for patients with N+ at diagnosis.
The boost volume encompasses areas of known disease with 1- to 2-cm margins.
Setup and Field Arrangement for Conventional Technique
An intraoral stent is used to open the mouth and depress the tongue. When surgical resection includes removal of the hard palate, the stent can be designed to hold a water-filled balloon to occlude the surgical defect (see Chapter 3). Orbital exenteration defect, if present, is filled directly with a water-containing balloon (see Case Study 12-3) or other types of bolus material.
The patient is immobilized in a supine position with a slight hyperextension of the head to bring the floor of the orbit parallel to the axis of the anterior beam. This position allows delivery of the desired dose to the orbital floor without irradiating through a large volume of the ipsilateral eye.
Marking of lateral canthi, oral commissures, and external scar facilitates portal design. When there is no external scar
(e.g., after craniofacial resection), a wire is placed on the premaxillary skin to indicate the slope of this structure. In addition, it is helpful to mark the position of the medial and inferior limbus with the eyes gazing forward for the purpose of corneal shielding. The location and size of the tumor determine the appropriate portal borders and arrangement.
(e.g., after craniofacial resection), a wire is placed on the premaxillary skin to indicate the slope of this structure. In addition, it is helpful to mark the position of the medial and inferior limbus with the eyes gazing forward for the purpose of corneal shielding. The location and size of the tumor determine the appropriate portal borders and arrangement.
Case Study 12-1
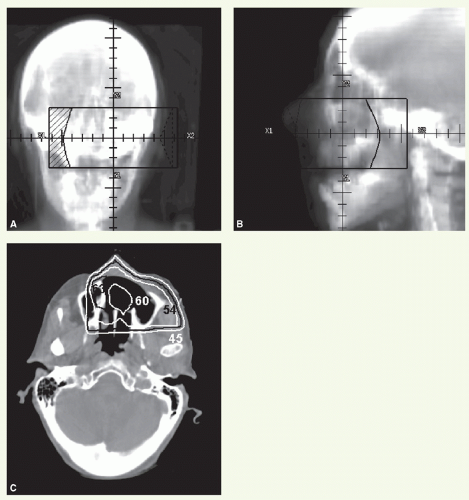
A 77-year-old woman presented with tingling of the left anterior maxillary gingiva. Physical examination showed a mass in the gingiva extending to the hard palate. Imaging studies revealed that the epicenter of the mass was in the inferior maxillary sinus, with extension to both the posterior wall and the premaxillary soft tissue. She underwent an infrastructure maxillectomy. Histologic examination revealed grade 2 mucoepidermoid carcinoma.
Figure 12.1 illustrated postoperative radiation delivered using anterior (Fig. 12.1A) and left lateral (Fig. 12.1B), wedgedpair, portals with 45-degree wedges. The total dose was 60 Gy, with a serial reduction made at 54 Gy. An isodose distribution through the resected sinus is shown in Figure 12C.
Case Study 12-2
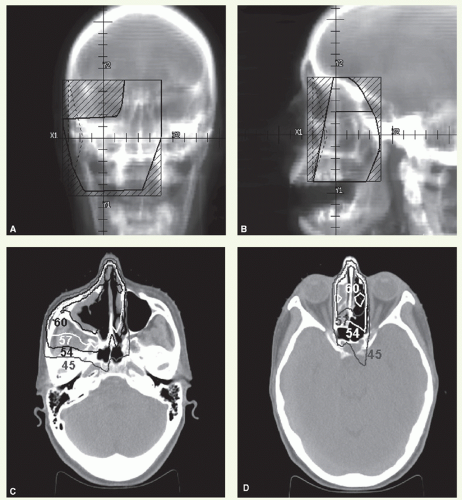
A 46-year-old woman was found to have a right nasal polyp, biopsy of which revealed neuroblastoma. Imaging revealed the bulk of tumor in the medial wall of the right maxillary sinus. She underwent a medial maxillectomy and postoperative radiation.
Figure 12.2 shows details of radiation treatment delivered through an anterior (Fig. 12.2A) and two lateral-opposed portals (Fig. 12.2B) with 6 MV photons, with 60-degree wedges being used on the lateral fields with the heels oriented anterior. The loading of anterior to lateral-lateral was 1:0.07:0.07. The lateral fields were reduced after 40 Gy, and the total dose was 56 Gy specified at the 95% line. Representative isodose distributions through the maxillary sinus and between the orbits are shown in Figure 12.2C,D.
Case Study 12-3
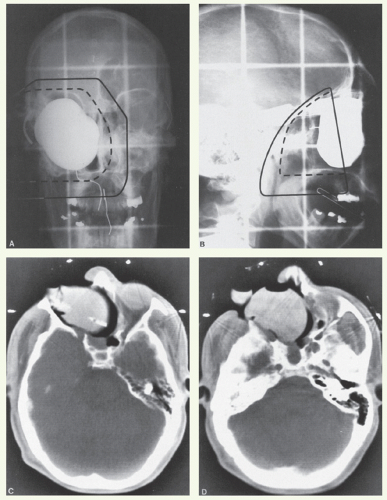
A 67-year-old man underwent a removal of a polyp of the right nasal cavity. Histologic examination showed an inverted papilloma. Three years later, he underwent a second polypectomy for recurrence. Histologic examination revealed a small focus of carcinoma within inverted papilloma. Eight months after the second surgery, this patient was referred to the M.D. Anderson Cancer Center for treatment of a large recurrence located in the right maxillary sinus, orbit, and infratemporal fossa. He underwent resection of this tumor with orbital exenteration. Histologic examination revealed inverted papilloma with multiple foci of squamous cell carcinoma.
The margins of resection contained papilloma but were free of invasive carcinoma. He received postoperative radiotherapy as shown in Figure 12.3. The surgical bed was treated with an anterior (Fig. 12.3A) and right (Fig. 12.3B) and left lateral fields loaded 1:0.15:0.15, respectively. A stent was used to depress the tongue. A water-filled balloon was placed in the surgical defect. The surgical scar, lateral orbital canthi, and oral commissures are marked. The initial target volume received a dose of 50 Gy in 25 fractions, specified at the isocenter. Subsequently, the fields were reduced to administer a boost dose of 10 Gy in 5 fractions to the tumor bed. A CT scan was obtained for treatment planning, which showed good filling of the surgical defect with the water-filled balloon (Fig. 12.3C,D). This patient did well until 22 months later when a pedunculated lesion was noted in the right ethmoid remnant along with a firm area in the floor of the maxillary defect. Biopsy of both lesions revealed diffusely infiltrating inverting papilloma with focal squamous cell carcinomas.
For tumors of the infrastructure with no extension into the orbit or ethmoids (uncommon), anterior and ipsilateral wedge-pair (usually 45-degree wedges) photon fields are used (Case Study 12-1). The use of the “half-beam” technique (i.e., placing the isocenter at the level of the orbital floor and shielding of the upper half of the fields) prevents exposure of the contralateral eye by beam divergence. Anterior portal borders:
Superior: just above the floor of the orbit but below the cornea.
Lateral: 1 cm beyond the lateral wall of the maxillary sinus (or falling-off when there is tumor extension into the facial soft tissues).
Medial: 1 to 2 cm across midline.
Inferior: 1 cm below the floor of the maxillary sinus or below the surgical bed.
Lateral portal borders:
Superior and inferior: same as the anterior portal.
Anterior: in front of the anterior wall.
Posterior: behind the pterygoid plates or more posteriorly depending on the extent of the contiguous tumor spread.
For tumors of the infrastructure spreading across midline through the hard palate, lateral-opposed photon fields are preferred. The use of the “half-beam” technique (i.e., placing the isocenter at the level of the orbital floor and shielding of the upper half of the fields) prevents exposure of the contralateral eye by beam divergence. The portal borders are similar to the lateral field described previously.
For tumors involving the suprastructure or ethmoids, a three-field technique is used (Case Studies 12-2 and 12-3). An anterior portal is combined with right and left lateral fields. Loading varies from 1:0.15:0.15 to 1:0.07:0.07 depending on the tumor location and photon energy. The lateral fields have 60-degree wedges and can have a slight posterior tilt. Anterior portal borders:
Superior: above the crista galli to cover the ethmoids and, in the absence of orbital invasion, at the lower edge of the cornea to cover the orbital floor. When the orbit is involved, an attempt is made to shield the lacrimal gland whenever possible to avoid occurrence of dry, painful eye. Tumor extension into the frontal sinus or cranial fossa calls for a more generous superior coverage.
Inferior: 1 cm below the floor of the maxillary sinus or below the surgical bed.
Medial: 1 to 2 cm, or farther, across midline to cover the contralateral ethmoidal extension.
Lateral: depends on the tumor extent (1 cm beyond lateral orbital wall when this structure is intact or falling off when there is tumor extension into facial soft tissues or infratemporal fossa).
Lateral portal borders:
Superior: follows the contour of the floor of the anterior cranial fossa.
Inferior: corresponds to that of anterior portal.
Anterior: behind the lateral bony canthus parallel to the slope of the face as marked by the wire.
Posterior: behind the pterygoid plates or more posteriorly, depending on the extent of the contiguous tumor spread and the surgery.
For boost volume, the portal size is reduced to encompass the tumor bed and to exclude as much optic pathway as possible. The contralateral optic nerve and chiasm are excluded from the field after a dose of 54 Gy in 27 fractions. Sometimes, this requires two field reductions (i.e., after 50 Gy and 54 Gy, respectively). When the lesion abuts these structures, the benefits and risks of delivering a maximum dose of 60 Gy in 30 fractions, which carries a 5% to 10% risk of blindness resulting from nerve injury, are discussed with the patient.
For treatment of the neck nodes, ipsilateral upper neck irradiation is given to patients with squamous cell or undifferentiated carcinomas, stages T2 to T4 NO. This is accomplished through a lateral appositional electron field.
Superior border: sloping up from the horizontal ramus of the mandible anteriorly to match the inferior border of the primary portal posteriorly. This portal matching creates a small triangle over the cheek, which is irradiated with an abutting triangular, appositional electron field (6 MeV) when there is tumor extension into facial soft tissues.
Anterior border: just behind the oral commissures.
Posterior border: at the mastoid process.
Inferior border: at the thyroid notch (above the arytenoids).
Bilateral neck treatment is indicated in patients presenting with palpable node(s). Proper field-matching technique should be selected in this setting to minimize dose heterogeneity, particularly to prevent overdosing in the depth by beam divergence. This can be accomplished by treating both the primary tumor bed and the upper neck with half-beam technique (shielding the caudal half of maxillary fields and the cephalad half of neck fields) to eliminate divergence and thereby prevent beam overlap. The central axis of the primary tumor portals and that of the opposed-lateral upper neck fields are placed at the axial plane of the inferior portal border of the maxillary fields (i.e., usually 1 cm below the floor of the maxillary sinus). It is prudent to move the junction line between the primary and neck fields during the course of treatment. The mid and lower neck is irradiated with an anterior appositional photon field matched to
the inferior border of opposed-lateral upper neck fields (see “General Principles”).
the inferior border of opposed-lateral upper neck fields (see “General Principles”).
The portal borders of the maxillary fields are as defined previously. The borders of the upper neck fields are determined by the extent of the nodal disease. If the initial lateral fields are on the spinal cord, portal reduction is made after approximately 45 Gy. The posterior cervical areas are then irradiated to the desired dose with abutting electron fields.
Intensity-Modulated Radiation Therapy Planning
The complex anatomy of the paranasal sinuses makes it appealing to use high-precision conformal radiotherapy for the treatment of sinonasal tumors to reduce normal tissue toxicity without compromising the dose to the tumor bed. IMRT generally yields better dose distribution for these tumors (see Case Studies 12-4 and 12-5).
The patient is immobilized in a supine position with an extended head and shoulder thermoplastic mask. Thin-cut computed tomography (CT) scans are obtained in treatment position. The clinical target volume (CTV) and planning target volume are outlined for dosimetric planning.
Virtual Gross Target Volume
There is no actual gross target volume (GTV) after a complete surgical tumor resection. However, it can be useful to formulate a virtual GTV (vGTV) to facilitate target volume definition. The vGTV is the best approximation of the tissues having high likelihood of harboring microscopic tumor reconstructed based on findings of preoperative clinical examination, imaging studies, and surgical-pathologic assessment. Bulky flaps can cause substantial distortions in the tumor bed and should, therefore, be taken into account in reconstructing the vGTV.
Clinical Target Volumes
Three CTVs are generally delineated.
CTVHD delineates volumes to receive the highest dose. This includes the primary and nodal vGTVs with 1-cm margins. The entire sinus is included in this volume. Medially, the ipsilateral nasal cavity to the septum is included. Generous coverage is given posteriorly into the residual masticator space and pterygomaxillary tissues as this is a frequent site of recurrence. The lateral edge includes the masticator space. In patients with partial palate resection, CTVHD includes at least 1 cm of the remaining palate. If the floor of the orbit was involved, CTVHD needs to cover the inferior orbital tissues as a minimum. For disease extension beyond the sinus, CTVHD should cover these tissues with a 0.5- to 1-cm margin.
CTVID delineates volumes to receive an intermediate dose. For the primary tumor bed, CTVID encompasses a 0.5- to 1-cm additional margin beyond CTVHD. For anterior tumors in particular, the skin, if not covered by CTVHD, will need to be included, and bolus may be required. For the neck, CTVID covers the dissected nodal region not harboring involved nodes.
CTVED delineates volumes to receive an elective dose for subclinical disease. When microscopic perineural invasion is present, the maxillary nerve up to foramen rotundum, if not covered in higher dose CTVs, should be encompassed in CTVED. For extensive perineural extension (involvement of large nerve or presence of clinical signs), CTVED includes the proximal V2 up to the trigeminal ganglion. In squamous cell or undifferentiated carcinomas without clinical nodal involvement, CTVED encompasses the ipsilateral nodal levels I and II and buccal and facial nodes. For tumors crossing midline, CTVED covers bilateral nodes.
Case Study 12-4
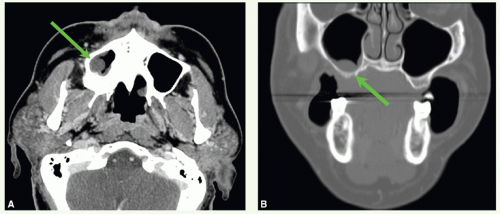
A 60-year-old woman presented with a loose right maxillary tooth. A biopsy taken from the tissue adjacent to the tooth was positive for squamous cell cancer (SCC).
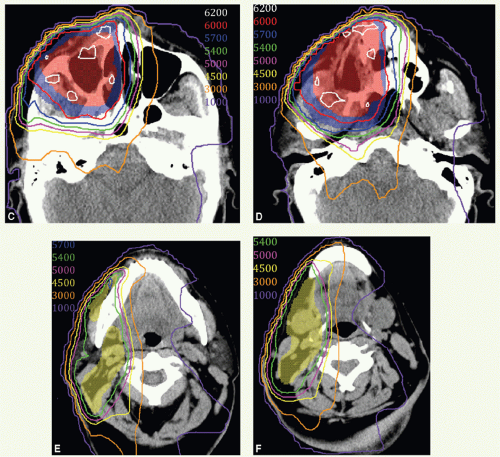
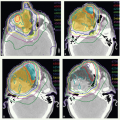
A CT scan was obtained and demonstrated a mass in the inferior aspect of the right maxillary sinus. The mass (green arrows) can be seen on axial (Fig. 12.4A) and coronal views (Fig. 12.4B). She underwent an infrastructure maxillectomy. Histologic examination revealed SCC with bone invasion (stage pT4 cNO).
Postoperative IMRT was administered in 30 fractions. CTVHD (red colorwash), CTVID (blue), and CTVED (yellow) were prescribed 60 Gy, 57 Gy, and 54 Gy, respectively. CTVHD encompassed the right maxillary sinus and medial aspect of the resected palate, CTVID an additional margin on the operative bed, and CTVED the undissected, ipsilateral lymphatics considered at risk including the right facial nodes and right level I and II nodes. Figure 12.4 displays contours and isodose distribution on axial views at the level of the mid sinus (Fig. 12.4C), inferior sinus (Fig. 12.4D), facial and superior level II nodes (Fig. 12.4E), and mid level I and II nodes (Fig. 12.4F). The patient was in an excellent general condition and had no evidence of disease at the last follow-up.
Case Study 12-5
A 58-year-old man presented with a right facial mass and oral pain.
A CT scan showed a large mass in the right maxillary sinus invading the right buccal space and soft tissues of the cheek (Fig. 12.5A,B




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree