Introduction
Approximately 10,500 cases of childhood cancer are diagnosed each year, which represent about 1% of all new cancers in the United States. Nearly 45% of these cancers will either be acute lymphoblastic leukemia or brain and other central nervous system (CNS) tumors. More than 80% of patients afflicted with childhood cancer will be alive at 5 years from initial diagnosis. In 2014, an estimated 1350 deaths occurred secondary to cancer in children 14 years of age or younger. About 25% of deaths in children who survive at least 5 years from their diagnosis die of treatment-related complications. Many of the late effects of treatment can be linked to the use of radiotherapy (RT). The reduction of RT dose has been implicated in the corresponding reduction in late toxicities, including secondary neoplasms.
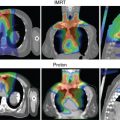
Proton therapy has the potential to reduce complications in children because of the reduction of integral dose to normal tissues when compared with photons. The ability of protons to spare normal tissues beyond a specified depth can be advantageous to the somatic and function of the organ or tissue beyond the target and may result in the reduction of secondary neoplasms.
Unique problems in children
The late effects of RT in children can be different from those in adults. Although the predominant manifestation of radiation injury in the adult will usually be fibrogenic or inflammatory, which contributes to loss of organ function, in children, there is an added clinical manifestation of growth delay and impaired maturation of the organ. Young children under 7 years of age are particularly vulnerable if the brain and musculoskeletal organs are irradiated because this is the time when these organ systems are growing and developing. Teenagers are particularly vulnerable to secondary neoplasms when the breast tissue is irradiated during puberty, resulting in a higher incidence of secondary breast cancer compared with irradiation of a young child or adult. Hence, strategies to eliminate or reduce RT during these vulnerable periods have been used. For children under 3 years of age with brain tumors, adjuvant chemotherapy has been used to delay the start of RT until the child is 3 years old. In ependymoma, for example, only 40% and 20% of children are progression-free at 2 years and 5 years, respectively, with this approach. , This dogma has been challenged by researchers from St. Jude Children’s Research Hospital, who have shown a 3-year progression-free survival (PFS) rate of 74.7% in 88 children with a median age of 2.9 years; neurocognitive testing showed stable cognitive findings, with more than half of the children tested at 2 years after RT. Currently, many young children with brain tumors to be treated with RT are referred for proton therapy to minimize late effects.
Proton therapy literature on children
Although the theoretical benefit of proton therapy is obvious, many in the pediatric oncology community have questioned whether protons have the same tumor control efficacy as photons. Others have questioned whether the reduction of integral dose will have a clinically significant effect on long-term toxicity when compared with very sophisticated photon plans possible using intensity-modulated radiation therapy (IMRT), volumetric arc therapy, and tomotherapy. Although few publications deal with protons and their effectiveness for tumor control, the current literature suggests that protons are equivalent to photons with regard to local control of tumors. Furthermore, literature is evolving to suggest that some acute and late effects can be minimized by using proton therapy.
Medulloblastoma
Patients with medulloblastoma have a lot to gain by not receiving radiation doses to organs anterior to the spine during the craniospinal portion of the treatment. Structures such as the thyroid gland and breast tissue are particularly prone to developing secondary cancers. Cardiovascular late effects after RT have also been reported for patients with medulloblastoma. Craniospinal doses for patients with average-risk and standard-risk disease are 23.4 and 36 Gy. The tumor bed is given an additional RT boost dose to a total dose of 54 to 55.8 Gy.
In a multiinstitution study of 88 patients with standard-risk medulloblastoma given chemotherapy and proton ( n = 45) or photon ( n = 43) therapy, the 6-year relapse-free survival rates were 78.8% and 76.5%, repectively, whereas the 6-year overall survival (OS) rates were 82.0% and 87.6% for protons and photons, respectively. Patterns of failure were similar among both groups. At MD Anderson, the 3-year event-free survival (EFS) rates were 77.0% and 53.0%, whereas the 3-year OS rates were 90.7% and 73.4% for standard ( n = 63) and high-risk ( n = 33) groups, respectively, which was similar to the historical photon literature.
A comparison of adult medulloblastoma patients treated with protons ( n = 19) or photons ( n = 21) revealed that the proportions of patients with more than 5% body weight loss ( P = .004), grade 2 nausea and vomiting ( P = .004), medical management of esophagitis ( P < .001), reduction of white count ( P = .04), reduction of hemoglobin ( P = .009), and reduction of platelets ( P = .05) were smaller with protons compared with photons.
A recent phase II trial of 59 patients treated with proton therapy for medulloblastoma revealed that the cumulative incidence of Pediatric Oncology Group (POG) grade 3 to 4 ototoxicity was 16% at 5 years. The full-scale intelligence quotient decreased by 1.5 points per year without a change in perceptual reasoning index and working memory. The cumulative index of any neuroendocrine deficit at 5 years was 55%. A recent comparison of grade 3 to 4 ototoxicity that used three different ototoxicity scales (POG, Brock, and International Society of Pediatric Oncology [SIOP – Boston]) showed essentially the same proportions of proton and photon patients developing hearing loss. Although protons led to a reduction in the RT dose to the cochlea relative to photons, this did not translate into hearing preservation, probably because patients also received cisplatin.
Hypothyroidism has also been shown to be less common among children receiving proton craniospinal irradiation (CSI). A retrospective comparison of standard-risk medulloblastoma patients treated with protons versus photons showed that the hypothyroidism rate was reduced from 69% to 23% with protons ( P < .001). Use of photons for CSI was also associated with a greater risk of sex hormone deficiency (19%–3%; P = .025), a requirement for any endocrine replacement therapy (78%–55%; P = .030), and a greater height standard deviation score difference compared with proton CSI. No differences were found in the incidence of growth hormone deficiency, adrenal insufficiency, or precocious puberty.
Ependymoma
As mentioned, RT is now routinely used to treat young children with ependymoma. Children who are at least 1 year old receive adjuvant RT to the tumor bed. The only exception for postoperative RT is for a child with a grade II supratentorial tumor that has undergone gross total resection. Investigators from Boston have reported comparable local control and PFS with protons compared with other photon series. The 3-year PFS rates were 88% and 54% for gross total and subtotally resected disease from using protons, respectively, and compares well with the St. Jude 5-year PFS rates of 91.5% and 41% for gross total and subtotally resected disease in children, respectively. At the Texas Children’s Hospital and the MD Anderson Proton Center, investigators compared the 3-year PFS rates for children with ependymoma treated with both RT modalities. The 3-year PFS rates were 82% and 60% for proton and photon patients, respectively ( P = .031); however, gross total resection was more common in the proton group than in the photon group (93% vs. 76%, respectively; P = .043).
Craniopharyngioma
In a retrospective comparison of 52 children with craniopharyngioma treated with protons and photons, researchers from the MD Anderson and the Texas Children’s Hospital showed no difference in cystic failure-free, nodular failure-free, and OS rates between the two groups. The 3-year cystic failure-free and nodular failure-free survival rates were 75.5% (67% protons, 76.8% photons) and 95% (91.7% protons, 96.4% photons), respectively.
Intracranial germ cell tumors
At the Massachusetts General Hospital, the 3-year local control, PFS, and OS rates for 22 patients with intracranial CNS germ cell tumors were 100%, 95%, and 100%, respectively. At the MD Anderson Proton Center, the 5-year local control, PFS, and OS rates for germinoma and nongerminomatous germ cell tumor (NGGCT) were 89%, 89%, and 100% for germinoma and 82%, 82%, and 82% for NGGCT, respectively. The above reports indicate that proton therapy does not compromise local control or PFS in intracranial germ cell tumors.
Low-grade glioma
At the Massachusetts General Hospital, 32 pediatric patients received proton therapy for low-grade glioma of the brain or spinal cord. The 6- and 8-year PFS rates were 89.7% and 82.8% with an 8-year OS rate of 100%, respectively. Some decline in neurocognitive function was seen in children irradiated when they were younger than 7 years. A higher dose to the left temporal lobe and hippocampus was also implicated in cognitive decline. The incidence of endocrinopathy was higher among those receiving more than 40 Gy (relative biological effectiveness [RBE]) to the hypothalamus, pituitary, or optic chiasm. The progression-free and OS rates above are similar to those in a series of 39 patients with low-grade glioma treated with IMRT, with 8-year PFS and OS rates of 78.2% and 93.7%, respectively.
Atypical teratoid/rhabdoid tumor
At the MD Anderson Proton Center, 31 children received proton therapy for atypical teratoid/rhabdoid tumor. The median age at the time of rhabdoid tumor was 24 months, with 17 receiving radiation to the primary site and 14 receiving CSI followed by primary site irradiation. With a median follow-up time of 2 years, the median PFS time was 20.8 months, and the median OS time was 34.3 months, which is comparable to the historical photon series. ,
Retinoblastoma
Proton therapy might be beneficial for children with retinoblastoma, as they are often very young; some with hereditary disease have a high chance of developing secondary tumors. In a retrospective analysis of 49 patients with retinoblastoma (84% with bilateral disease), the local control rates with proton therapy were high. The enucleation rate was 11% for International Classification for Intraocular Retinoblastoma (ICIR) stage A to B disease and 23% for ICIR stage C to D disease. In a prospective study of 12 children with bilateral retinoblastoma treated with protons, none of the patients had hormonal deficiencies. Facial hypoplasia was less common after proton therapy relative to after enucleation. Patient and parent-proxy quality of life were not severely affected by proton therapy. An analysis of proton- versus photon-treated children with retinoblastoma found that none of the patients who received protons had an in-field secondary malignancy, whereas 14% of patients treated with photons developed in-field tumors ( P = .015). The 10-year cumulative incidence rates of secondary tumors (in-field and out of field) were 5% for protons and 14% for photons ( P = .12).
Rhabdomyosarcoma
In a phase II study from researchers at the Massachusetts General Hospital and the MD Anderson Cancer Center, 57 children with low- and intermediate-risk rhabdomyosarcoma received proton therapy. The 5-year local control, event-free, and OS rates were 81%, 69%, and 78%, respectively. At the University of Florida, 66 children with rhabdomyosarcoma received proton therapy with a 2-year local control rate of 88%. All of the local failures were within the 95% isodose line. Several dosimetric studies of rhabdomyosarcoma at various body sites have shown a theoretical benefit from protons by reduction of doses to surrounding normal tissues. To date, however, no clinical findings have been reported to confirm improvements in toxicity from protons.
Ewing sarcoma
A retrospective review of 30 patients with Ewing sarcoma treated with proton therapy at Massachusetts General Hospital revealed a 3-year local control, EFS, and OS of 86%, 60%, and 89%, respectively. The 3-year actuarial rates of EFS, local control, and OS were 60%, 86%, and 89%, respectively.
Chordoma/chondrosarcoma
Although protons have been used to treat chordomas and chondrosarcomas with excellent results, most studies have included mostly adults, with only two publications including primarily children. At the Paul Scherrer Institute, 19 patients with chordoma and 7 with chondrosarcoma received proton therapy, with 5-year local control rates of 81% and 80%, respectively. At the Institut Curie Proton Center in Orsay, 26 children with skull-base and cervical chordoma received proton therapy, with a 5-year local control rate of 81%.
Neuroblastoma
Proton therapy was given to 21 sites in 14 patients with advanced neuroblastoma at the University of Tsukuba. The 3-year locoregional control rate was 82%. At the University of Pennsylvania, 13 patients underwent proton therapy, with 5 patients receiving treatment to more than 2 sites. At a median follow-up time of 16 months, there were no local failures at irradiated sites. At the Massachusetts General Hospital, nine children with high-risk neuroblastoma received proton therapy. At a median follow-up time of 38 months, there were no locoregional failures. These results compare favorably with locoregional control rates reported after photon therapy.
Wilms tumor
A dosimetric study comparing protons to the tumor bed only versus photons given in parallel opposed anteroposterior/posteroanterior fields showed more sparing of normal tissues with the approach using protons. However, because the target volumes were much smaller in the proton patients, this finding is expected. If the tumor bed alone is considered the target, then proton beam therapy will have an advantage in reducing the dose to normal tissues anterior to the tumor bed.
Patient selection for proton therapy
Which pediatric patients are best suited for treatment with proton therapy? In general, patients who are most likely to gain from proton therapy are those with curable tumors and with tumors that are near critical structures that are not part of the target volume. Children with localized tumors will probably have more benefits relative to children with metastatic disease. A consensus conference on proton therapy for children was recently held in Stockholm and identified the following tumors as being the best candidates for proton therapy: low-grade glioma, optic pathway tumors, intracranial germ cell tumors, medulloblastoma, ependymoma, craniopharyngioma, pineal tumors, chordoma/chondrosarcoma, rhabdomyosarcoma, Ewing sarcoma, and retinoblastoma. For neuroblastoma and lymphoma, the experts were noncommittal for either modality, whereas for high-grade glioma of brain and brainstem and Wilms tumor, most favored photon therapy. The expert participants also questioned the value of proton therapy for cases requiring total body, whole abdominal, whole lung, and whole brain RT. Neutron contamination generated by proton scatter, which may result in a higher rate of secondary neoplasms, was not considered a major barrier to the use of proton therapy.
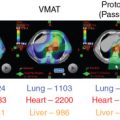
The magnitude of benefit for proton therapy should also be considered when proton centers prioritize cases. Children receiving CSI will likely have more benefit with protons because of the sparing of heart, lungs, thyroid gland, abdominal organs, and gonads from the exit dose of the photon spinal field. For other patients, the magnitude of benefit may be weighed against the cost of treatment, including the patient’s family moving for 2 months to cities where proton therapy is available. Proton therapy is still a scarce commodity in the United States and worldwide; it not uncommon for patients to travel across state lines or to different countries to receive protons.
With the number of proton centers in the United States increasing from 2004 to 2013, the number of children treated with proton therapy have followed the same trend. Currently, about 17.5% of children requiring RT receive proton therapy. A recent study of the National Cancer Database found that children receiving protons tend to be younger, have insurance, and have higher median household income and higher levels of education.
Summary
Proton therapy is a good option for the radiotherapeutic management of many curable types of childhood cancer. The current data suggest equivalence in local control, progression-free, and OS for many childhood tumors. Based on single-institution prospective and retrospective studies, proton therapy may be able to reduce certain acute and late toxicities of RT. In a group of patients likely to survive the disease, the reduction of late effects is paramount for maintaining a good quality of life.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree