Pediatric venous occlusions are a growing cause of morbidity and mortality, especially in hospitalized patients. Catheter-directed recanalization is a safe and effective treatment option in appropriately selected patients. Benefits of catheter directed therapies (CDTs) include the prevention of pulmonary embolism and end organ failure acutely as well as superior vena cava syndrome and post-thrombotic syndrome chronically. Timely diagnosis, recognition of underlying factors for thrombosis, and familiarity with the spectrum of tools and techniques for CDT are essential to optimizing outcomes in the acute setting. Recanalization of chronic venous occlusions can similarly provide symptomatic relief and achieve long term vessel patency. This review will detail the scope, techniques, and outcomes for CDT in the treatment of acquired systemic deep vein occlusions.
Introduction and background
Pediatric venous occlusion is a significant cause of hospital-related morbidity that can lead to fatal outcomes. The incidence of venous occlusion ranges from 0.07 to 0.49 per 10,000 children, with a more than 10-fold increase in hospitalized children. Mortality occurs primarily from thromboembolic disease. Systemic deep vein thromboses (DVTs) can lead to these occlusions and are most often provoked by central venous catheters (CVCs). These catheters are routinely placed via a tunneled femoral approach in neonates resulting in a higher incidence of inferior vena cava (IVC) thrombosis. Conversely, catheters in older children are placed over the chest or upper extremities affecting the subclavian vein, brachiocephalic vein, and superior vena cava (SVC). De novo thrombotic occlusions more commonly occur in the renal veins in neonates (nephrotic syndrome), subclavian and iliac veins in older children (Paget Schroetter and May-Thurner Syndromes), SVC in all age groups and lower extremities in syndromic patients (Klippel-Trenaunay Syndrome and CLOVES Syndrome). Anticoagulation is the first line management for patients with deep vein thrombosis, however pharmacomechanical thrombolysis, thrombectomy, and stenting may be indicated in specific scenarios. Acquired systemic venous occlusions of the vena cava, brachiocephalic, iliofemoral, and renal veins will be discussed in this review including indications for treatment, goals of care, technical considerations to optimize success, and strategies to minimize complications. Congenital occlusions will not be discussed in this review.
Indications
Strategies for recanalization depend on occlusion chronicity, extent, location, pathology, and patient size. A detailed history of prior thrombosis in the patient or family members and laboratory evaluation for a hypercoagulable condition are imperative to predict long-term outcomes once a treatment has been selected. Acute thrombus is defined as having formed within 7-14 days of the procedure—often an estimated timeframe based upon duration of symptoms. There is no equivalent temporal cut-off to distinguish sub-acute and chronic thrombus. The main goals for interventional treatment of DVTs are the prevention of pulmonary embolism (PE) in the acute setting and SVC or post-thrombotic (PTS) syndromes in the chronic setting.
Progressive head and neck-related symptoms of venous obstruction arising from narrowing and occlusion of the SVC or central thoracic veins may manifest acutely or chronically and are an indication for interventional therapy. Common symptoms of SVC syndrome include head and neck swelling, distended collateral veins extending over the chest, dyspnea, cough, orthopnea, mental status changes, chylothorax, and large pleural effusions. Less commonly identified symptoms include scholastic underperformance and delayed milestones for patients with mild symptoms. SVC syndrome can be fatal if left untreated with recanalization often providing immediate relief.
Occlusion of the IVC can similarly are, however collateral venous flow is often present in the majority of cases and patients are only treated if symptomatic. Symptoms include PTS, which typically manifests as pain, persistent swelling, edema, and skin wounds with poor healing. CDT should ideally be performed during the acute phase to improve benefit. Care should be taken with patient selection to establish individual bleeding risks (such as in trauma patients) and avoid additional morbidity. Pre- and post-procedure assessment of symptoms should incorporate a validated scoring system such as the clinical, etiology, anatomy, and pathophysiology (CEAP) score.
Propagation of renal vein thrombus (RVT) is another cause of IVC occlusion and thromboembolism. RVT was once considered a medical emergency managed with nephrectomy, however has been managed conservatively since the 1960s. Despite trends towards anticoagulation and fibrinolysis, greater than 70% of patients experience irreversible renal damage. Heparin anticoagulation is indicated to prevent renal failure in neonates with bilateral RVT and can treat other underlying causes for thrombosis such as factor V Leiden thrombophilia. , CDT and thrombectomy can similarly be effective in preserving renal function and preventing acute clinical decline in patients with bilateral RVT and RVT extending into the IVC, suggesting a combination of anticoagulation and CDT is indicated in these groups. , Older patients with acute symptoms of flank pain, hematuria, recurrent RVT and renal dysfunction may benefit from CDT regardless of the laterality or extent.
External compression of central veins represents an additional pathology that can lead to complete venous occlusion. Paget Schroetter Syndrome (upper extremity subclavian thrombosis due to extrinsic rib, scalene, or ligamentous compression) and May-Thurner Syndrome (left external iliac vein compression by the right external iliac artery) are important pathologies to identify as they lead to recurrent thromboses and occlusion if the area of compression is not properly treated. Anticoagulation is used to treat limited acute DVTs peripheral to the subclavian vein or below the inguinal ligament while thrombectomy and thrombolysis are reserved for more central occlusions to prevent PTS.
Imaging evaluation
Cross-sectional imaging prior to any planned procedure demonstrating a lack of venous return, collateral venous drainage, large PEs, new PEs while receiving anticoagulation, and clinical signs and symptoms of venous occlusion are indications to proceed with interventional treatment. Imaging review must include assessment for external mechanical compression that may cause recurring clot burden with or without anticoagulation.
Initial imaging of renal occlusions and many pelvic occlusions begins with ultrasound (US) examination. Findings include decreased or absent flow on color Doppler exam, a lack of compressibility, and absent spectral waveform. The blunting or absence of an augmentation response on color Doppler imaging or decrease in respiratory phasicity relative to the contralateral iliofemoral vein may indicate upstream venous obstruction, although the utility of augmentation has been questioned. ,
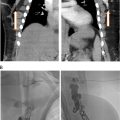
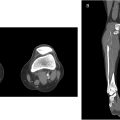
CT and MR are used after US screening to detail the extent of occlusion, illustrate vascular anatomy, and help plan intervention. The lack of ionizing radiation and dynamic imaging of MR are often weighed against the spatial resolution of CT ( Fig. 1 ).

Procedure
Positioning
Patients undergoing recanalization of the vena cava, thoracic veins, or renal veins are positioned supine. Patients undergoing recanalization of pelvic venous occlusions may alternatively have DVT propagation into the lower extremities that can affect patient positioning depending on whether an antegrade popliteal vein approach (prone) or retrograde jugular approach (supine) is used. Treatment may require single or dual access depending on the location of occlusion. Renal occlusions are often approached from an internal jugular (IJ) vein approach given the acute angle of the veins as they connect to the IVC in neonates and children. The fluoroscopy unit should be positioned so that cross-sectional imaging, such as cone beam CT (CBCT), or intravascular ultrasound (US) can be used. One of the accesses should always be made from an antegrade approach to allow for intermittent venograms during crossing.
Access
Once the patient has been sterilely prepped, venous access is obtained with a 21-gauge needle using a 15 MHz or higher frequency US probe and a transitional dilator. The dilator is exchanged over an intermediate or stiff 0.35” wire for a vascular sheath. Required sheath sizes range from 4 French to 24 French depending on the recanalization device and stents used. Antegrade access to thoracic occlusions must be high enough in the neck or arms to allow adequate room to exchange needles for standard sheaths and catheters. Careful US planning should be performed prior to access to ensure the vein provides a direct route to the occlusion, as many tortuous collateral vessels arise that can mimic the internal or external jugular veins. When utilizing an IJ approach for pelvic thromboses, longer sheaths are recommended for stability. A sheath length of 40 cm is usually appropriate in children under the age of 10 while 65 cm is sufficient in older patients. Sheaths longer than 10 cm should be connected to a pressurized infusion bag of heparinized normal saline.
Acute recanalization
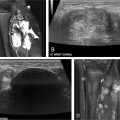
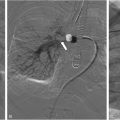
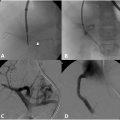
Recanalization and reconstruction techniques are similar to those used in adult IR procedures, but attention to pediatric vessel size and the long-term durability of implants are important considerations. Acute thrombus can usually be crossed with an angled catheter and a regular or stiff 0.35” Glidewire (Terumo, Tokyo, Japan). Probing the occlusion with a Glidewire from an antegrade direction is the initial strategy for thoracic occlusions. When approaching renal and pelvic occlusions from the neck, a venogram is necessary to exclude extension of renal thrombus and left common iliac thrombus into the IVC or contralateral vein if no preoperative cross-sectional or ultrasound imaging is available. Crossing techniques vary and are uniformly different from those used for acute arterial occlusions. The formation of a wire loop while probing an occlusion is useful in traversing the clot and maintaining wire position within the vessel lumen ( Fig. 2 ). Unlike arteries, veins are not prone to dissection from these wire loops. Care should be taken to maintain wire position along the expected course of the vessels. Multiple contrast venograms may be required to ensure the catheter and wire have not perforated a vessel. Fluoroscopic visualization of the renal contour in younger patients serves as a guide to determining the endpoint of wire probing, which is necessary as renal vein recanalization often involves establishing proximal intraparenchymal patency to limit atrophy. Alternatively, transabdominal ultrasound can be used to image the IVC and pelvic veins in children under 1 year of age ( Fig. 3 ). Intravascular ultrasound (IVUS) is useful in older children particularly for pedunculated RVT extension into the IVC ( Fig. 4 ). When the catheter has reached the level of the heart, peripheral kidney or thigh, contrast injection by hand should be performed to assess for patent inflow and collateral vessels. Digital subtraction venography is not needed during the initial injection given the partial or complete occlusion of the vessel and the potential to cause perforation of veins or tamponade in cases of pericardial perforation.



Chronic recanalization
When crossing CTOs, 2 access sites are often needed from antegrade and retrograde directions. While weighted wires may be used to cross chronic calcified occlusions in small vessels, 0.035” guidewires are used in older children. Re-entry devices are not needed when crossing venous CTOs as opposed to arterial CTOs. Alternatively, CTO crossing in infants and younger patients may be performed with an access needle in select cases. Percutaneous US-guided sharp recanalization is utilized primarily in younger children to CTOs in veins near the skin surface ( Fig. 5 ).