Venous malformation (VM) stands as the most prevalent form of vascular malformation, characterized by its diverse morphology. These lesions can manifest in any part of the body, affecting different tissue planes and giving rise to symptoms such as pain, swelling, or physical dysfunction. In the realm of treatment, direct puncture VM sclerotherapy holds its place as the primary approach. This technique involves the administration of a sclerosing agent into the VM channels during contrast phlebography while simultaneously managing the outflow veins through different methods. The process of VM sclerotherapy induces endothelial damage, thrombosis, and fibrosis, resulting in symptom relief through lesion shrinkage. It is crucial to exercise caution techniques and sclerosing agents during VM sclerotherapy to minimize procedural complications, enhance clinical outcomes, and ultimately improve the patient’s overall quality of life.
Introduction
Venous malformation (VM) is the most common vascular malformation, characterized by the abnormal development of the vein wall. This condition involves thinning and asymmetrical disruption of the smooth muscle layer of the affected vein, along with abnormalities in endothelial cells. The morphological features of VMs vary depending on the affected tissue or organ and the specific type of vein involved. Treatment options for VMs encompass compression, resection, and embolization/sclerosant injection to obliterate the VM channels. However, compression therapy alone is seldom sufficient, prompting patients to seek additional interventions to alleviate symptoms. Sclerotherapy is typically the preferred initial treatment for VM due to its noninvasive nature and patient tolerance. The goal of VM sclerotherapy is to induce a cascade of endothelial damage, thrombosis, and fibrosis, leading to symptom reduction through lesion shrinkage. Commonly employed sclerosing agents for VMs include Sodium Tetradecyl Sulfate (STS), Ethanol, Polidocanol, and Bleomycin. STS acts as a detergent sclerosant, directly damaging the endothelial lining of the VM. Absolute ethanol has demonstrated exceptional effectiveness, particularly for large VMs. However, its aggressive nature can lead to serious complications such as extensive swelling, tissue necrosis, peripheral nerve injury, hemolysis, pulmonary vasospasm, and cardiac arrest. Therefore, ethanol should only be administered by trained and experienced interventionalist within a hospital setting with ample support from anesthesiology and intensive care units (ICUs). , In critical locations near the airway or orbit, where other sclerosing agents may cause significant swelling and compromise airway or optic nerve integrity, bleomycin can be utilized for VM treatment. ,
Clinical evaluation of the patient
History and physical
VMs can be identified by their bluish skin discoloration and compressible nature upon examination. When the affected area is positioned dependent, the VM may exhibit swelling. Additionally, performing the Valsalva maneuver, which increases peripheral venous pressure, can further contribute to VM swelling. VMs do not exhibit pulsation but have a soft and compressible texture upon palpation. , In any cases of initial vascular anomalies clinic visit, multiple specialties should be involved to make a correct diagnosis and discuss treatment strategies.
Imaging
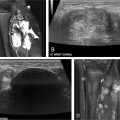
Ultrasound (US) and Magnetic Resonance Imaging (MRI) play pivotal roles as imaging modalities in the diagnosis and evaluation of VM. , , US is often employed as the initial imaging modality due to its effectiveness in identifying superficial malformations. It offers practical advantages, as it does not require sedation and involves no radiation exposure. US provides valuable information regarding the size and complexity of the VM, as well as the presence of associated clots or phleboliths. On the other hand, MRI is considered the gold standard imaging technique for VMs. It offers exceptional spatial resolution, enabling comprehensive visualization of the distribution of VMs across all tissue layers, including bone, joints, muscles, tendons, and nerves. MRI serves a crucial role in confidently diagnosing VMs while excluding other potential vascular malformations or tumors. Prior to any interventional or surgical procedures, MRI is recommended as a baseline study for future follow-up and appropriate treatment planning.
Laboratory tests
The majority of small, focal VMs do not require any laboratory tests. However, conducting a thorough hematologic evaluation at the time of VM diagnosis is crucial particularly when dealing with extensive lesions prior to initiating treatment. Fibrinogen and D-dimer levels are essential for large or diffuse VMs as they can serve as indicators of localized intravascular coagulation (LIC). This is crucial to identify and address promptly, as LIC has the potential to progress into disseminated intravascular coagulation (DIC), especially in cases involving aggressive sclerotherapy , Prophylactic anticoagulation is recommended for patients with high-risk factors such as extensive and large VMs to mitigate the risk of thrombosis. This can involve the administration of low-molecular-weight heparin or direct-acting oral anticoagulants (DOACs), accompanied by frequent monitoring of fibrinogen and D-dimer levels. Close surveillance is necessary before and after any sclerotherapy procedure or surgery to ensure optimal patient management and minimize the complications associated with thrombosis. , ,
Indications for the procedure
There are absolute and relative indications, as well as contraindications, to consider when determining the need for VM treatment. , , Absolute indications encompass situations such as hemorrhage, VMs located in life- or limb-threatening areas, or those posing a risk to vital functions. Relative indications include progressive and debilitating pain or discomfort, functional impairment impacting daily activities or quality of life, severe cosmetic deformity accompanied by psychological distress, and VMs located in high-risk areas prone to secondary complications. However, certain relative contraindications must be taken into account. These include the presence of nearby nerve trunks that may be susceptible to treatment-related damage, significant connections to deep veins potentially increasing the risk of thromboembolic disease, extensive involvement of the skin, the presence of consumption coagulopathy, and chronic pulmonary embolic disease. It is essential to carefully assess these factors to ensure appropriate treatment decisions and minimize potential risks for the patients.
Sclerosing agents
Various sclerosants can be employed, depending on the type of VMs. Suitable sclerosants include ethanol, detergent sclerosants like STS, Polidocanol, sodium morrhuate, ethanolamine oleate, and bleomycin.
Absolute ethanol (95%-98%) is an aggressive sclerosant that causes instant precipitation of endothelial cell proteins and rapid thrombosis. Ethanol can result in transmural vessel necrosis and diffusion into the surrounding tissues occurs. It is the most effective sclerosant available, especially in large VMs, but it also results in the most serious side effects, including massive swelling with possible compartment syndrome, tissue necrosis, and peripheral nerve injury, hemolysis, pulmonary vasospasm, and cardiac arrest. Therefore, ethanol should be avoided when dealing with lesions adjacent to major nerves or cutaneous lesions.
Detergent sclerosants are commonly used for VM sclerotherapy because of their relative safety and comparable outcome to ethanol. STS is one of the most popular detergent sclerosants, and it is used as a 1% or 3% solution and can be combined with air to create foam, which can be made radiopaque by mixing it with iodinated contrast or ethiodized oil. Ethiodized oil also enhances viscosity and facilitates deeper penetration of STS into the VM channels with a longer duration of foam. In the case of 3% STS, the volume should be limited to below 0.5 mL/kg per session.
Bleomycin has historically been a proven effective treatment of lymphatic malformations but is a potent therapeutic option in VMs and may be more effective than other agents in specific cases. Bleomycin is particularly useful for mucosal lesions or regions susceptible to swelling, such as the orbit or airway.
Procedural steps
VM sclerotherapy can be performed on an outpatient basis. Most patients, especially children require general anesthesia. Some adult patients may undergo the procedure with conscious sedation or local anesthesia as long as the VM is not located in a critical area and ethanol is not part of the treatment plan.
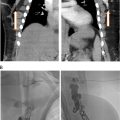
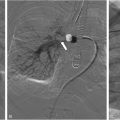
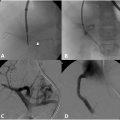
The process of VM sclerotherapy involves percutaneous access to the malformed VM channels using a 20-25-gauge needle or angiocatheter with US guidance. Intralesional digital subtraction angiography (DSA) or roadmap fluoroscopy imaging is necessary to confirm the needle’s position in the VM channel, its connection to adjacent veins, and the estimation of the VM’s capacity by assessing the injected contrast volume. Given the slow flow nature of VMs, a DSA rate of 1 frame per second is sufficient, and the iodinated contrast can be diluted with an equal amount of normal saline to reduce the total amount of contrast. It is crucial not to exceed the VM channels’ capacity to avoid the risk of VM rupture or needle dislodgement.
Once the needle’s position in the VM channel is confirmed, the sclerotherapy procedure can commence. When using STS foam, the ratio of STS, contrast, and air may vary based on the interventionalist’s preference. In our practice, we use a ratio of STS:contrast:air = 2:1:1, and the foam is created by agitating the mixture using a 3-way stopcock. The injection of STS foam can be monitored with US and roadmap fluoroscopy imaging to prevent extravasation or egress into deep veins. Additional STS foam may be injected until the lesion is completely filled, as evaluated by fluoroscopy and US ( Figure 1 ).