Neuroimaging, particularly positron emission tomography (PET), plays a crucial role in diagnosing and managing brain disorders by providing insights into diverse neuropathologies such as vascular issues, infections, inflammation, degenerative diseases, and tumors. In dementia, [18F]FDG-PET helps predict Alzheimer’s disease (AD) development from mild cognitive impairment, revealing metabolic reductions in specific brain regions. PET’s evolution with novel radiotracers and advanced imaging techniques addresses diagnostic challenges and enhances disease monitoring. Despite limitations like off-target binding, PET remains indispensable in clinical neurology, offering noninvasive insights into brain functions, disease progression, and treatment responses.
Key points
- •
Short-Term Dementia Prediction in MCI: 18 F-FDG PET, focusing on posterior cingulate cortex metabolic activity, predicts dementia development in mild cognitive impairment (MCI) individuals, surpassing amyloid PET’s predictive efficacy.
- •
AD Subtypes’ Metabolic Patterns: 18 F-FDG PET reveals distinct hypometabolism patterns corresponding to Alzheimer’s disease (AD) subtypes like PCA, bvAD, and lvPPA, aiding subtype diagnosis based on symptomatic features.
- •
Enhanced Tau PET Imaging: Recent tau PET advancements, exemplified by [ 18 F]-flortaucipir, improve tau pathology visualization in AD and related disorders, with ongoing efforts toward second-generation radiopharmaceuticals addressing diagnostic limitations.
- •
Huntington’s Disease and Chorea-Related Conditions: HD shows striatal hypometabolism, seen also in neurochoreacanthocytosis syndromes, SCA17, and Wilson’s disease. Conversely, acquired chorea conditions like Sydenham’s chorea exhibit increased striatal metabolism on [ 18 F]FDG-PET.
- •
18 F-FDG PET in Epilepsy: Crucial for nonlesional epilepsy or multifocal lesions. Helps differentiate mesial versus lateral temporal lobe epilepsy but less effective for extratemporal epilepsy (45%–92% sensitivity for frontal lobe). Ictal FDG-PET not generally recommended due to complex interpretations, but exceptions exist for frequent seizures. Integration with PET/MR imaging enhances diagnostic capabilities.
Introduction
Neuroimaging plays a crucial role in managing brain disorders by aiding in making accurate diagnoses or offering clinically relevant alternative diagnoses ( Table 1 ). The wide range of neuropathologies, such as vascular issues, infections, inflammation, degenerative diseases, and tumors, presents diverse clinical manifestations that can lack specificity. Therefore, neuroimaging is essential for developing effective management strategies by providing clarity in diagnosis and guiding treatment plans.
| Disease | FDG PET/CT Pattern |
|---|---|
| Mild cognitive impairment | Reductions in metabolic activity in the posterior cingulate cortex. |
| Alzheimer’s disease | Decreased metabolic activity in parietotemporal association cortices, posterior cingulate cortex, and precuneus with relative preservation in primary cortices. |
| Behavioral variant AD | Early frontal lobe involvement, challenging to differentiate from behavioral variant frontotemporal dementia. |
| Posterior cortical atrophy | Hypometabolism in posterior brain regions, particularly affecting the visual cortex. |
| Logopenic variant primary progressive aphasia | Hypometabolism in left temporoparietal junction, associated with language deficits. |
| Frontotemporal dementia | Reduced metabolism in frontal and anterior temporal lobes. |
| Dementia with Lewy bodies | Hypometabolism in occipital cortex, with sparing of posterior cingulate cortex. |
| Huntington’s disease (HD) | Predominant striatal hypometabolism. |
| Neurochoreacanthocytosis syndromes | Similar to HD, striatal hypometabolism. |
| Spinocerebellar ataxia 17 | Striatal hypometabolism, similar to HD. |
| Wilson’s disease | Striatal hypometabolism, similar to HD. |
| Sydenham’s chorea | Increased striatal metabolism. |
| Multiple system atrophy | Decreased metabolism in the striatum (especially posterior putamen), cerebellum, and brainstem. |
| Progressive supranuclear palsy | Reduced metabolism in midbrain and frontal cortex. |
| Corticobasal degeneration | Asymmetric hypometabolism involving the cortex and basal ganglia. |
Positron emission tomography (PET) is an advanced imaging technique designed to utilize compounds labeled with positron-emitting radioisotopes for visualizing biochemical and molecular characteristics within living organisms. The application of molecular imaging through PET encompasses activities such as determining the biological origins of diseases and evaluating the effectiveness of treatments. Glucose is transported from the bloodstream into the brain through glucose transporters (GLUTs), with GLUT1 primarily expressed in the blood–brain barrier and astrocytes and GLUT3 expressed in neurons. Inside the cells, glucose is converted to glucose-6-phosphate by hexokinase and then enters the glycolytic pathway, leading to the production of adenosine triphosphate. However, deoxyglucose-6-phosphate, a derivative of glucose-6-phosphate, cannot undergo further glycolysis. This is because the activity of glucose-6-phosphatase, the enzyme responsible for reversing the conversion of glucose-6-phosphate back to glucose, is low in the brain. As a result, deoxyglucose-6-phosphate accumulates within cells. Studies have revealed that glucose is transported from capillaries to astrocytes, where it is metabolized into lactate. This lactate then acts as an energy source for neurons, a phenomenon known as the “astrocyte-neuron lactate shuttle.” FDG-PET has certain limitations, particularly in neuro-oncology imaging. The naturally high baseline glucose metabolic rate in normal brain tissue can result in the underdetection of low-grade tumors or the recurrence of high-grade tumors after treatment. However, since the introduction of fluorodeoxyglucose (FDG), the US Food and Drug Administration (FDA) has approved several new PET radiotracers in the last decade. PET provides a noninvasive approach for studying brain functions in vivo. It enables quantification of neuroreceptor binding, cerebral metabolism, and blood flow. PET is becoming more prevalent in clinical neurology as it assists in diagnosing diseases in early or ambiguous cases and tracks disease progression and treatment response. This article delves into the primary applications of PET in clinical neurology.
PET/MR imaging
Integrating neuroimaging techniques such as magnetic resonance imaging (MR imaging) and PET has significantly enhanced our understanding of dementia, particularly Alzheimer’s disease (AD). MR imaging techniques like diffusion tensor imaging and functional MR imaging allow us to investigate structural and functional connectivity networks implicated in dementia, such as the default mode network. These networks involve regions like the medial prefrontal cortex, angular gyrus, and posterior cingulate cortex, shedding light on how brain connectivity changes in dementia. PET imaging, especially with the advent of amyloid PET radiotracers like [11C]-Pittsburgh compound-B (PIB) and fluorinated tracers, has revolutionized our ability to visualize and quantify β-amyloid plaque pathology, a hallmark of AD. By examining the patterns of brain metabolism, PET imaging can help differentiate between various clinical subtypes of dementia and provide crucial insights into disease progression and potential treatment avenues.
The integration of these imaging modalities offers a comprehensive approach to dementia diagnosis and treatment planning. Clinicians can benefit from the wealth of information provided by both structural and molecular imaging techniques, leading to more accurate diagnoses and personalized treatment strategies. Additionally, the ability to acquire multiple modalities in a single imaging session is particularly advantageous for elderly or frail patients, minimizing discomfort and streamlining the diagnostic process. Overall, the synergy between MR imaging and PET imaging holds great promise for advancing our understanding of dementia and improving patient care outcomes.
Dementia
Mild Cognitive Impairment
One of the notable benefits of 18 F-FDG PET is its ability to predict the development of dementia in individuals with mild cognitive impairment (MCI) over the short term. Specifically, reductions in metabolic activity in the posterior cingulate cortex have been shown to possess high predictive value in this regard. Studies have demonstrated that 18 F-FDG PET can offer additional predictive value beyond amyloid PET imaging. Specifically, research has shown that individuals with MCI who are positive for amyloid but do not exhibit hypometabolic abnormalities in 18 F-FDG PET tend to maintain clinical stability over several years. This highlights the importance of metabolic assessments in combination with amyloid imaging for more accurate prognostication in MCI patients.
Alzheimer’s Disease
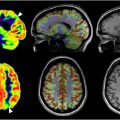
AD is a neurodegenerative condition with an unclear cause and pathogenesis. It predominantly impacts older individuals and stands as the leading cause of dementia. A key and frequently initial symptom of AD is selective memory loss, although there can be variations in presentation. In 2018, the NIA-AA committee introduced the first biological definition of AD, shifting the diagnostic focus from symptoms to the presence of amyloid plaques (A) and tau tangles (T). This approach also utilized neurodegeneration biomarkers (N) to aid in disease staging. The system categorized clinical stages from 1 to 6: stage 1 indicated biomarker changes only, stage 2 involved subtle cognitive changes, stage 3 represented MCI, and stages 4 to 6 denoted mild, moderate, and severe dementia, respectively. These criteria were designed to evolve over time and integrate new data as it became available. The primary application of 18 F-FDG PET in a dementia assessment is to distinguish between AD, frontotemporal dementia (FTD), and dementia with Lewy bodies (DLB). Researchers have extensively characterized the 18 F-FDG PET imaging features associated with AD, FTD, and DLB over the past 4 decades. Various neurodegenerative disorders exhibit selective vulnerability, meaning they tend to affect specific brain regions while sparing others to some extent. Analyzing the spatial patterns of decreased 18 F-FDG uptake compared with relatively preserved uptake in the brain provides valuable clues about the specific neurodegenerative substrate underlying the observed changes. Various neurodegenerative disorders exhibit selective vulnerability, meaning they tend to affect specific brain regions while sparing others to some extent. Analyzing the spatial patterns of decreased 18 F-FDG uptake compared with relatively preserved uptake in the brain provides valuable clues about the specific neurodegenerative substrate underlying the observed changes. In AD, there is commonly decreased metabolic activity in the parietotemporal association cortices, along with the involvement of the posterior cingulate cortex and precuneus ( Fig. 1 ). The distinct metabolic phenotype of AD is characterized by a comprehensive regional pattern of metabolic impairment, particularly notable in the posterior cingulate and temporoparietal cortices. These deficits are often more pronounced compared with frontal cortex involvement. Furthermore, this metabolic phenotype is defined by the relative preservation of metabolic activity in regions such as the primary sensorimotor and visual cortices, basal ganglia, and cerebellum. However, metabolic activity in areas such as the primary sensorimotor and primary visual cortices, as well as the basal ganglia, thalamus, pons, and cerebellum, tends to be relatively preserved.

As AD progresses, there is typically involvement of the frontal association cortex ( Fig. 2 ). However, in a subset of AD patients with pronounced behavioral symptoms, known as the behavioral variant of AD (bvAD), frontal lobe involvement can be detected early in the disease course. This early frontal lobe involvement in bvAD can make it challenging to differentiate from the behavioral variant of frontotemporal dementia (bvFTD) using 18 F-FDG PET imaging. Silverman and colleagues conducted a study evaluating the use of 18 F-FDG PET for diagnosing AD compared with other causes of dementia. The study included 2 populations: a prospective cohort with a long-term clinical follow-up and a retrospective cohort with a histopathologic reference standard. The retrospective cohort involved multiple clinical facilities internationally that collected both brain 18 F-FDG PET and histopathologic data from patients being assessed for dementia. Among 97 patients with a histopathologic diagnosis of AD, the sensitivity of 18 F-FDG PET for diagnosing AD was 94% (with a 95% confidence interval [CI] of 89%–99%), and the specificity among 41 patients without AD was 73% (95% CI, 60%–87%). The study also examined a subset of patients with mild disease at the time of PET and found that the performance of 18 F-FDG PET in terms of sensitivity (95%), specificity (71%), and overall diagnostic accuracy (89%) was nearly identical to that of the entire group. Different subtypes of AD are associated with distinct patterns of hypometabolism observed on 18 F-FDG PET scans. These metabolic patterns closely mirror the symptomatic features seen in patients with AD subtypes. AD encompasses various subtypes with distinct clinical presentations: Posterior Cortical Atrophy (PCA): This subtype is characterized by visual symptoms and deficits. PCA typically manifests with dominant visual-constructive deficits as an initial clinical feature. Behavioral Variant of Alzheimer’s Disease (bvAD): This subtype is associated with frontal executive and behavioral symptoms, as discussed earlier in the article. Logopenic Variant Primary Progressive Aphasia (lvPPA): This subtype is characterized by language-dominant deficits, as discussed later in the article. AD and vascular dementia (VaD) are 2 prevalent causes of dementia that frequently occur together, a condition known as “mixed dementia.” The prevalence of mixed dementia can be quite high, especially in elderly individuals. As new pathologic markers are developed, there is growing recognition of mixed dementia with the coexistence of multiple neurodegenerative disorders alongside vascular changes. This acknowledgment is particularly prominent in elderly patients. These subtypes demonstrate unique clinical features that correlate with specific patterns of hypometabolism observed on 18 F-FDG PET scans, aiding in their differentiation and diagnosis. These observations also suggest that in the later stages of AD, different subtypes may converge toward a common pattern of disease topography.


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree