Portal interventions in pediatric patients present unique difficulties when compared to adult procedures. In addition, children who need a portal intervention require a different workup and clinical management. Based on these elements, the clinical decisions for the study and treatment of these pathologies are different. This review is intended to present a summary of the interventional radiologist’s role in treating pediatric portal venous diseases. Focus is placed on the technical elements, patient management and procedural indications while discussing different interventions involving the portal vein, providing some recommendations supported by recent research and the authors’ experience.
Many diverse etiologies may have a negative impact on the portal vein system and therefore determine the need for a portal vein intervention to solve or at least palliate those effects.
Although the causes of portal vein disease in the pediatric population are different from adult patients, therapeutic IR procedures follow the same steps. This article will focus on the modifications in the materials and techniques needed between the adult and pediatric population.
Clinical manifestations arise from 2 main mechanisms: portal hypertension (resulting in splenomegaly, low platelet count, ascites, malabsorption, and gastro-esophageal bleeding) and portal shunting (growth retardation, encephalopathy, hepato-pulmonary syndrome). ,
Portal interventions in children are frequently indicated in the setting of cavernomatous disease, cirrhosis, liver transplant complications and congenital portosystemic shunts.
We will discuss the most frequently required procedures to treat portal vein disease and give basic guidelines to perform these procedures in the pediatric population.
Postliver transplant portal vein angioplasty and recanalization
Portal vein stenosis and thrombosis are frequently related to liver transplant complications Portal vein stenosis has a prevalence of 1%-10% in transplant recipients. The peak incidence of thrombosis involves the first 30 days after liver transplant and has an incidence between 5%-20%.
Clinical manifestations of portal vein stenosis or occlusion include portal hypertension, ascites, gastro-intestinal bleeding, splenomegaly, platelet count drop, and hepatic failure.
The first imaging approach should include a Doppler US exam, where a maximum velocity at the portal anastomosis 180 cm/s is highly suggestive of stenosis. The evaluation is completed with complete blood count (CBC), coagulation evaluation, an MR angiogram or CT angiogram, which give the anatomical information to plan the following therapeutic procedure. The presence of a significant stenosis on imaging studies or persistent clinical signs with a suggested stenosis on imaging indicate the need for a percutaneous portal angioplasty.
Percutaneous transhepatic angioplasty is the preferred treatment for portal vein stenosis. However, if intrahepatic portal branches are not suitable for access, a trans-splenic access can be used. This approach is increasingly used for these and many portal procedures, demonstrating to be safe and effective.
As per most percutaneous portal access procedures, the first step involves the transhepatic US guided puncture of a portal branch with a 22G needle. Micropuncture sets are advised in pediatric patients. Portography can be performed through the micropuncture set and then exchanged for a 6 or 7Fr vascular sheath. A hydrophilic coated 0.035” guidewire and a 4 Fr angiographic catheter are used to pass through the stenosis and is later exchanged for a stiff guidewire to perform angioplasty. , , Angioplasty alone is usually sufficient, and stenting is reserved for patients with recoil evidence in the postangioplasty venogram, recurrent stenosis, or when the stenosis involves an interposition graft.
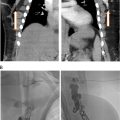
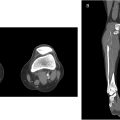
If portography shows a complete occlusion of the portal vein, recanalization can be attempted. The first steps usually consist of trying to cross through the obstruction with a guidewire (our first option is often a hydrophilic coated 0.035” wire) and catheter (usually a short 4Fr Kumpe angiographic catheter). If this does not work, switching to different guidewires, or a splenic approach (in case the procedure was being attempted through a transhepatic access) are valid options. More recently, we are performing portal vein recanalization through a trans-splenic approach as the first option. If all of these fail a sharp recanalization can be attempted using the back end of a wire ( Fig. 1 ).

When portal vein thrombosis occurs during early posttransplantation then surgical thrombectomy, or even retransplantation, is usually the first choice. If thrombosis occurs at a later period, endovascular thrombectomy can be attempted. Techniques for percutaneous thrombolysis include thrombus aspiration, angioplasty and transcatheter infusion of recombinant tissue plasminogen alteplase (rtPA) (0.1mg/Kg). , ,
Procedure-related complications have an incidence between 1% and 5%. These include abdominal and gastrointestinal bleeding, biliary tract bleeding, biliary fistula, peritonitis, sepsis, and restenosis. To avoid bleeding access complications we always perform tract embolization with Gelfoam pledgets and/or coils. Vascular plugs can also be used for this purpose. This technique significantly reduces bleeding risk.
After the procedure, the patient must follow an anticoagulation/antiplatelet aggregating regimen. Regular follow-up consults including clinical, laboratory, and Doppler US are recommended for early detection of restenosis. The rate of restenosis after percutaneous angioplasty has been reported as up to 29%, with a good response to a second session.
Splenic artery embolization
Hypersplenism is a hematologic syndrome associated with portal hypertension caused by an enlarged and overactive spleen. It’s characterized by thrombocytopenia (64%-84%), leucopenia (5%), neutropenia and anemia.
Partial splenic embolization has been proposed as an alternative for a high morbidity surgical splenectomy. This procedure not only reduces the spleen size and therefore hypersplenism, but also reduces splenic outflow while maintaining partial splenic immune function.
Besides the standard preprocedure lab tests, patients must have their vaccinations completed to cover encapsulated bacteria. This is especially important in infants and will help to palliate the immunity deficit caused by functional partial splenectomy.
The procedure itself is a straightforward embolization with some precautions that must be followed to avoid the most severe complications. Through a femoral vascular sheath (3-5Fr according to patient size), selective splenic artery catheterization with a Cobra catheter is performed. A first angiogram will help to visualize and advance the catheter distally from the pancreatic and gastric branches which arise from the splenic artery.
Partial splenic embolization can be performed through the angiographic catheter itself or coaxially with a 0.027” microcatheter. Embolization can be performed using a selective catheterization approach (only a few targeted distal branches are completely embolized), or a nonselective embolization (embolic materials are injected more proximally at the splenic hilum). In both approaches parenchymal phase angiogram is used to estimate the percentage of embolization.
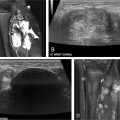
It has been demonstrated that hypersplenism relapses when the embolization percentage is under 50%, and different reports evidence a rise in serious complications (spleen necrosis and sepsis) when the embolization affects over 70% of the parenchyma. Many authors propose multiple sessions with 35%-50% of embolized territory (with 6-8 week intervals) to reduce postprocedural pain and risk of liquefactive necrosis. , , When performing selective embolization, the lower pole of the spleen should be targeted to prevent irritation of the diaphragm, pleural effusion, pneumonia or atelectasis, or intractable hiccups ( Fig. 2 ).

The most frequently used embolic agents are Gelfoam pledgets, polyvinyl alcohol (PVA) particles and gelatin microspheres. In our practice, we use 300-500 µm acrylic spheres, to achieve distal embolization. The injection of these agents in suspension with contrast agent and antibiotics (ampicillin-gentamycin) reduces the risk of splenic abscess formation. ,
Reports on splenic artery embolization report a significant (50%-80%) decrease in GI bleeding episodes in the adult population. There is also an improvement in liver function and a normalization of CBC.
Common major complications are pleural effusion, ascites, portal vein thrombosis, and splenic abscess. These can be avoided by following the procedural precautions mentioned above. Postembolization syndrome (fever, left upper abdominal pain, nausea, and perisplenic fluid) is reported in as high as 73% of patients. Post procedural care should include an aggressive analgesic plan, antibiotic prophylaxis and antiemetics. , While admitted, follow-up Doppler US should be perfomed to assess splenic and portal vein patency, and repeat CBC obtained.
Angioplasty of surgical portosystemic shunts
Surgical shunts for the alleviation of portal hypertension are preferred over TIPS when the patient has good liver function, and a hepatic transplant is not planned in the near future. These include a meso-Rex shunt and a variety of less selective portosystemic communications, including splenorenal, splenocaval or mesocaval shunts.
While portosystemic shunts have a high initial technical success rate, they suffer from a relatively high rate of stenosis, occlusion (2%-40%), and recurrent stenosis (20%). ,
Clinical evidence of portal hypertension or elevated peak anastomotic velocities on Doppler US indicate the need for a venography and endovascular intervention which could help to extend the shunt patency. Contrast enhanced CT or MR angiogram may help the radiologist to choose the appropriate approach.
For the treatment of a meso-Rex shunt, transhepatic right portal branch access or splenic access is preferred (depending on preprocedural imaging). While stenosis of the meso-Rex shunt may be encountered on either side of the anastomosis, the hepatic side is more frequently affected.
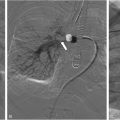
In the case of a dysfunctional splenorenal shunt trans-femoral or trans-jugular accesses are preferred due to its lower complication rate. Distal splenorenal shunts may be a challenge to engage. In this case, withdrawing a large reverse curve catheter (eg Simmons II) without the guidewire, from the distal left renal vein, usually helps finding the anastomotic ostium ( Fig. 3 ).


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree