Endovascular intervention is a safe, effective treatment modality in the management of diverse pulmonary vascular pathologies, including acute or chronic thromboembolic disease, pulmonary arteriovenous malformations (pAVMs), pulmonary artery or bronchial artery hemorrhage, and foreign body retrieval. This article reviews indications, contraindications, techniques, and outcomes in endovascular management of common pulmonary vascular pathologies, with the goal of improving operator familiarity and facility with these procedures.
Key points
- •
Pulmonary vasculature is susceptible to a range of pathologies, including thromboembolic, inflammatory, infectious, neoplastic, traumatic, congenital/hereditary, and iatrogenic diseases.
- •
Minimally invasive endovascular techniques have largely supplanted surgical management of many pulmonary vascular diseases.
- •
A thorough understanding of the indications, contraindications, evidence for, and technical approaches to pulmonary vascular interventions is the key in ensuring safe, effective endovascular management of pulmonary vascular diseases.
Introduction
Critical functions of the lungs include gas exchange and blood filtration. These roles are facilitated by a network of pulmonary arteries and pulmonary veins, with bronchial arteries providing additional vascular supply to major airways. The presence of multiple vascular beds renders the lungs susceptible to diverse pathologies, including acute or chronic thromboembolic disease; infectious, inflammatory, or traumatic injuries; pulmonary arteriovenous malformations; and foreign body embolization. With improvements in endovascular techniques, endovascular management of these pathologies has either supplanted or plays an important complementary role to surgical interventions. This article reviews indications, contraindications, techniques, and outcomes in endovascular management of common pulmonary vascular pathologies, with the goal of improving operator familiarity and facility with these procedures.
Pulmonary digital subtraction angiography
Though more recently supplanted by computed tomography (CT), pulmonary digital subtraction angiography (DSA) has long been the gold standard for characterizing pulmonary artery (PA) anatomy and pathology and remains a critical diagnostic step in many PA interventions. Pulmonary DSA also plays an important role in preoperative planning for PA operations such as pulmonary endarterectomy. In some settings, the ability to measure PA pressures (PAP) is critical in guiding therapy.
Contraindications to pulmonary DSA are few. Pre-existing left bundle branch block is a relative contraindication, as right heart catheterization can induce transient right bundle branch block. The risk of complete heart block in these patients can be mitigated with temporary pacing systems that can be activated if needed. Patients may present with severe pulmonary hypertension (PH), precluding moderate sedation, but the procedure may be performed with minimal sedation or local anesthesia only. Pulmonary DSA is classified as low bleeding risk by the Society of Interventional Radiology (SIR), with a platelet threshold of greater than 20,000 and international normalized ratio (INR) less than 2.0 to 3.0. The procedure can be done safely even with full anticoagulation, which should be continued in patients with history of thromboembolism.
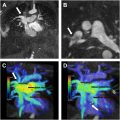
Pulmonary DSA begins with internal jugular (IJ) or common femoral vein (CFV) access in standard ultrasound-guided Seldinger fashion. After sheath placement, a flush catheter and wire are used to catheterize the main, right, or left PA (depending on procedural indication) under fluoroscopic guidance. PAP measurements can be obtained, followed by DSA. Projections for angiography (biplane frontal and lateral, ipsilateral posterior oblique) and non-ionic contrast injection rates vary by clinical condition ( Fig. 1 A-F ). ,

Diagnostic pulmonary angiography is well-tolerated. Minor arrhythmias are common when traversing the right ventricle, but major arrhythmias requiring intervention are uncommon. Regardless, continuous cardiac monitoring and defibrillation equipment must be available. In 707 consecutive patients undergoing diagnostic pulmonary arteriography over 4 years, minor to moderate complication rates (including transient angina, access site hematoma, and urticaria) were 1.4%, with only 1 major reported complication (inadvertent femoral artery injury requiring operative repair).
Catheter-directed management of pulmonary embolism
Acute pulmonary embolism (PE) is an important contributor to morbidity and mortality in the United States. Acute PE can be stratified into massive, submassive, or low-risk categories depending on clinical variables, including the presence or absence of sustained hypotension, imaging or electrocardiography (EKG) evidence of right ventricular dysfunction, and biochemical evidence of myocardial strain. Massive, submassive, and low-risk PEs have short-term mortality rates of 25% to 65%, 3% to 15%, and less than 1%, respectively, even if treated.
Low-risk PE may be managed with therapeutic anticoagulation alone. Massive PEs and, in some circumstances, submassive PEs require treatment to re-establish pulmonary perfusion and relieve right heart strain. Treatment has historically included therapeutic anticoagulation and/or systemic thrombolysis. Endovascular therapies that reduce or bypass thrombolytic administration, such as catheter-directed thrombolysis (CDT) or thrombectomy, have provided new tools in the treatment of massive and submassive PE. Full review of the evidence supporting thrombectomy and CDT is beyond the scope of this article, but multiple studies have demonstrated promising improvements in hemodynamic parameters and imaging evidence of right heart strain, though adequately-powered, randomized trials are lacking. Based on these results, catheter-based thrombectomy is recommended for massive PE requiring immediate intervention before thrombolysis can take effect, after failed thrombolysis, or in patients with contraindications to thrombolysis.
Contraindications to endovascular PE treatments vary by technique employed. Contraindications to CDT include active bleeding; recent major trauma, surgery, or intracranial injury; intracranial or spinal neoplasm; stroke within 6 months; and bleeding diatheses. Mechanical thrombectomy is more reasonable in these circumstances. The SIR classifies PE interventions as high bleeding risk, with goal platelets greater than 50,000 and INR less than 1.5 to 1.8, though anticoagulation should be continued if already initiated. As patients may present with tenuous hemodynamics and as catheter-based therapies may induce arrhythmias, endovascular PE interventions at the authors’ institution are performed with anesthesiology assistance.
Catheter-based PE intervention begins with CFV access, favored over IJ access as this facilitates navigation of larger-bore thrombectomy devices. After placement of an appropriately-sized sheath, main PA catheterization, pressure measurements, and diagnostic angiography are performed as described in this article’s first section. Intervention can then be pursued at the discretion of the treating interventionist.
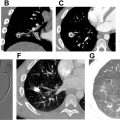
If mechanical thrombectomy is pursued, the venous access sheath is upsized, and a coaxial thrombectomy device is advanced over a wire until the target embolus is reached ( Fig. 2 B and E ). A bolus dose of therapeutic heparin (50–100 units/kg) is given during device insertion, as large-bore devices may otherwise induce thrombus. Available devices include manual vacuum catheters (FlowTriever Aspiration System, Inari Medical, Irvine, CA; AlphaVac, Angiodynamics, Latham, NY) or mechanized aspiration pumps (Lightning/Indigo Aspiration System, Penumbra Inc, Alameda, CA) ( Fig. 2 B and E). Multiple passes may be made with each device before removal. Pulmonary DSA and PAP measurements are performed following thrombectomy to confirm angiographic decrease in thrombus burden and/or reduction in PAP ( Fig. 2 C and F).

If catheter-directed thrombolysis is instead desired, single or dual infusion or flush catheters can be positioned central to or across the regions of embolus. Often, a bolus of tissue plasminogen activator (tPA) is given through each catheter, followed by tPA infusion via the lysis catheters and heparin infusion via the sheath sidearm. The patient returns for repeat arteriography within 24 hours, with catheter removal if thrombolysis is successful. If significant embolus burden remains, infusion catheters may be left in place for an additional 24 hours, though tPA infusions greater than 48 hours are not recommended. Enhanced catheter-directed thrombolysis devices, such as the EKOS ultrasonic CDT (USCDT) catheter (Boston Scientific, Marlborough, MA), which combines thrombolysis with high-frequency ultrasound, or the Bashir endovascular catheter (Thrombolex Inc, New Britain, PA), which combines pharmacologic thrombolysis with mechanical clot maceration, may alternatively be used.
Catheter-based PE therapies are associated with low complication rates, though complications vary by treatment type. CDT and USCDT carry an increased risk of bleeding (1.8% and 5%, respectively) relative to therapeutic anticoagulation. , Major bleeding is less likely in patients undergoing mechanical thrombectomy, with reported rates of 0.9% to 1.6%. However, large-bore mechanical thrombectomy devices predispose to other complications, including PA or cardiac injury or cardiovascular collapse (0.8%–1.0%); 30-day mortality estimates range from 0.8% to 1.0%.
Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension
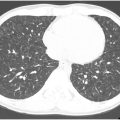
Chronic thromboembolic pulmonary hypertension (CTEPH) arises when acute pulmonary thromboembolism evolves into chronic intravascular scar, leading to chronic precapillary pulmonary hypertension (PH) (mean PAP ≥25 mm Hg and PA wedge pressure ≤15 mm Hg). While uncommon, with an incidence of 0.4% to 6.2% after pulmonary embolism, CTEPH is a source of prolonged morbidity after acute PE. The diagnosis of CTEPH is imaging-driven; ventilation-perfusion scans show significantly mismatched perfusion, and pulmonary DSA and pulmonary arterial-phase chest CT demonstrate vessel irregularities, outpouchings, intraluminal webs, stenoses, or occlusions.
While anticoagulation and medical management is critical in CTEPH care, pulmonary thromboendarterectomy (PEA) is the only definitive treatment. In patients with proximal thromboembolism and acceptable comorbidities, PEA is indicated. However, balloon pulmonary angioplasty (BPA) is a promising alternative to surgery in select patients who are not operative candidates due to distal clot burden or severe comorbidities, or in those with residual PH after PEA. Multiple trials have shown that BPA improves 6-min walk distance, mean PAP, functional class, and quality of life in patients relative to medical management. , Management decisions are complex, and patients should be referred to a specialized CTEPH center for multidisciplinary evaluation.
Contraindications to BPA include large, central thromboemboli, for which angioplasty is likely ineffective. , Patients with parenchymal fibrosis supplied by target vessels are unlikely to benefit from endovascular intervention. BPA is considered a high-bleeding- risk procedure with goal platelets greater than 50,000 and INR less than 1.5 to 1.8, though anticoagulant medications should be continued intraprocedurally. , , BPA should be performed with little to no sedation, as anesthetics can cause cardiovascular collapse in this population. ,
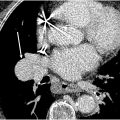
BPA is performed under fluoroscopic guidance. Venous access and PA catheterization is performed as described earlier, with CFV access preferred for ease of operation. Following systemic heparinization, target pulmonary branch arteries are reached via a sheath and coaxial guiding catheters, through which selective DSA can be performed ( Fig. 3 A ). Lesions should be targeted with the goal of treating the largest perfusional deficits, but morphology on DSA can further guide treatment. , Once a target vessel is identified, a microwire is advanced across the lesion. Distal balloon angioplasty begins with small-diameter (often 2.0 mm), compliant balloons; proximal segments may be treated with sequentially larger balloons, though these should be smaller than the native target vessel diameter. , Treatment endpoints are based on improvements in regional pulmonary blood flow on pulmonary DSA and corresponding decreases in pressure ( Fig. 3 B and C). Procedures may be repeated in multiple sessions, depending on the patient’s reserve and anatomic complexity. ,

While BPA outcomes were initially limited by reperfusion pulmonary edema in greater than 60% of patients, technical refinements have reduced complications, including wire injury, vessel dissection or rupture, parenchymal hemorrhage or hemothorax, hemoptysis, and pulmonary edema, to rates of less than 10% at experienced centers, with mortality rates of 3.9%. ,
Pulmonary arteriovenous malformation embolization
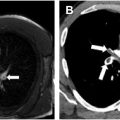
Pulmonary arteriovenous malformations (pAVMs) consist of abnormal direct connections between pulmonary arteries and pulmonary veins, creating right-to-left shunts. A total of 70% to 90% of individuals presenting with pAVMs have underlying hereditary hemorrhagic telangiectasia (HHT), though pAVMs may be sporadic or acquired secondary to trauma, cirrhosis, amyloidosis, or infection. ,
Patients with pAVMs are often asymptomatic, though dyspnea and fatigue may be presenting symptoms. Symptom severity corresponds with pAVM size and degree of right-to-left shunting, which can affect oxygenation and result in paradoxic stroke, transient ischemic attack, and cerebral abscess. Rationale for pAVM treatment even in asymptomatic patients is prevention of neurologic complications, other manifestations of paradoxic embolization, and (rarely) pulmonary hemorrhage. While historical guidelines advocated for treatment of AVMs with feeding vessels larger than ≥ 3 mm, newer recommendations advocate for first-line endovascular intervention in all accessible pAVMs. , , Resection or lung transplantation is reserved for rare cases of diffuse pAVM. Multiple observational studies have demonstrated high technical success rates, durable clinical success, and ischemic stroke rate reduction when state-of-the-art embolization techniques are employed. ,
In addition to the contraindications to pulmonary DSA described at the beginning of this article, relative contraindications to pAVM treatment include complex or diffuse pAVMs not amenable to endovascular management. pAVM embolization is considered a high- bleeding-risk procedure, with goal platelets greater than 50,000 and INR less than 1.5 to 1.8.
pAVM embolization is performed under moderate sedation or general anesthesia, with the goal of facilitating breath-holds. Care should be taken to reduce paradoxical embolism and stroke risks, including use of intravenous line bubble filters, intraprocedural heparinization, catheter double-flushing, and removal of guidewires under saline baths. After PA catheterization, base and selective arteriography is performed in multiple projections to delineate the target pAVM ( Fig. 4 B and C ). After feeding artery selection, embolization is performed using permanent embolic devices such as detachable coils or plugs. Embolization beginning within the sac or nidus is preferred over embolization of the feeding vessel alone , ( Fig. 4 D). Liquid or particle embolics should never be used, as these pose an unacceptable risk of paradoxic embolization. Post-embolization angiography is performed to ensure embolization of all feeding vessels ( Fig. 4 D). Multiple pAVMs may be treated in 1 session, though limiting treatment to a single lung is advocated by some practitioners. Following treatment, noncontrast or contrast-enhanced chest CT or bubble-study echocardiography may be used for surveillance.