Quality Assurance and Quality Control
Debra Deibel
Introduction
Digital mammography is still undergoing an evolution. The quality control (QC) for these systems is still being worked out and is vendor-specific. The reader is advised to use the methods recommended by the vendor until general QC measures are developed. It is also likely that digital breast tomosynthesis will replace conventional digital mammography, and this will offer a whole new set of QC challenges. This chapter deals with QC for conventional film/screen systems and has not been changed from the second edition of Breast Imaging.
Quality Control and Assurance for Film/Screen Mammography
A mammogram is among the most technically demanding of radiographic procedures. The early detection of breast cancer relies on the radiologist’s ability to perceive subtle changes that are perceptible only with high-quality imaging. Appropriately designed and adjusted equipment, proper positioning to maximize tissue visualization, proper exposure, and optimal film processing are the keys to obtaining high-quality mammograms. If the information is not contained on the study, accurate interpretation is obviously impossible.
Good technique requires constant attention to detail. This begins with the selection of the equipment to be used. Although screening must be low in cost to make its benefits available to all segments of the population, this does not mean low in quality. The screening mammogram may be the only opportunity to interrupt the course of the disease, and thus the screening study must be of the highest quality. As the number of women studied increases, the savings from the purchase of inferior equipment becomes insignificant, whereas the use of such equipment may compromise the detection of early lesions. With the passage of the Mammography Quality Standards Act (MQSA) by the U.S. Congress and its implementation by the Food and Drug Administration (FDA), basic quality assurance (QA) and QC are now a matter of law.
Quality must be monitored and maintained throughout the imaging sequence. The x-ray system should be routinely monitored for tube output and beam quality, with attention to resolution and contrast along with patient dose. Automatic exposure control should have a linear response and with rare exception should be used at all times to reduce the number of under- and overpenetrated studies. The screens in screen/film combinations should be routinely checked and cleaned to avoid artifacts that might compromise the image.
Quality Assurance
The goal of mammography is to image all the breast tissue with high contrast and high resolution at the lowest dose with as little noise as possible to permit the detection and diagnosis of breast cancer at as small a size and early a stage as possible. If screening is to be available to all, this must be done at a reasonable cost. QA specifies the overall management program, which includes policies and procedures designed to optimize the control of the performance of the facility and its equipment. Ultimately, the physician is responsible for implementing a QA program. QA requires an entire system of monitoring that not only ensures that the images are obtained and processed properly, but that
their analysis and interpretation are organized and monitored and that the information derived is accurately and effectively conveyed to the patient and her physician.
their analysis and interpretation are organized and monitored and that the information derived is accurately and effectively conveyed to the patient and her physician.
It is advantageous to use reporting systems that provide an organized structure for reporting and a standardized reporting language, such as the American College of Radiology (ACR) Breast Imaging Reporting and Data System (BIRADS). Computer systems are strongly recommended to facilitate the tracking of patients, especially those needing follow-up, to be certain that suggested measures have been taken when needed and that “the loop” has been closed. By collecting detailed statistical information such as the number of biopsies recommended and the number of cancers diagnosed, the cancer detection program can be monitored, adjusted, and improved.
Quality Control
QC is the segment of the overall QA program that specifies and implements measurements of the mammographic procedure to detect any variations from the optimum so that corrective actions can be taken promptly (1). Quality maintenance through QC monitoring of the production of the image is the foundation of an early detection program. The sequence of elements that must function properly to produce a high-quality mammogram can be considered an imaging chain. A weak link or break in the chain at any point compromises the effectiveness of early detection. All components should be optimized and, once optimized, monitored to be sure that optimization is maintained.
Equipment Acceptance Testing
A requisite for an effective QA program is to establish that all components of the imaging system are compatible and work well together. At the heart of the system is the mammography unit itself. Each site should have a medical physicist who performs a series of tests on new equipment to ensure that the equipment meets the manufacturer’s specifications, the expectations of the end user, and any mandated requirements. In addition, these acceptance tests qualify the system and provide a baseline for subsequent QC monitoring.
QC testing only ensures that the image quality is consistent with previous images without necessarily indicating the overall quality of the image. Thus, establishing an optimized baseline is the first step in a QC program. The various components and parameters of a QC program will likely vary over time as technologies evolve. The ACR publishes guides concerning these procedures (2).
Establishing The Components of Quality Imaging: An Optimized Baseline
Acceptance testing by the medical physicist, with guidance from the radiologist, should be designed to ensure the elements of image quality, patient dose, and radiation safety. The physicist should check the following:
Mammography unit assembly evaluation (e.g., detents, locks, indicators)
Collimation: Does the centering light field coincide with the radiation field?
Focal spot size measurements (both large and small focal spots)
Accuracy and reproducibility of kilovolt (peak) and timer stations
Beam quality assessment (half-value layer) done at kilovolt (peak) used clinically
Automatic exposure control: reproducibility, kilovolt (peak) compensation, minimum response time, backup timer verification
Uniformity of intensifying screens
Breast entrance exposure and average glandular dose
Compression pressures and mechanics
Tube leakage
Mammography phantom image baseline
Grid/artifact evaluation
A processor sensitometric strip baseline
These tests are documented in both the ACR Manual for Mammography Physicists and in the American Association of Physicists in Medicine Report No. 29. Any problems should initiate a call to the service engineer to take corrective action.
Mammography Equipment
Detector
Screen/film combinations that are specifically designed and suited for mammography must be chosen. At present this involves a single intensifying screen, used as a back screen, with spatial resolution sufficient to detect microcalcifications 2 to 300 μm in size, and properly coupled with a single-emulsion film sensitive to the emission spectrum (the color of light emitted) of the intensifying screen. The cassette containing the screen and film should have very low x-ray attenuation and high durability and provide intimate contact between the film and screen. In addition, the cassette should be designed so that the edges of the film and screen are positioned tightly against the chest wall edge of the cassette to minimize the amount of breast tissue that is not imaged.
Screen/Film Contact
One cause of image blur is poor contact between the screen and the film. The cassettes should be tested by imaging a 40-mesh copper screen to ensure good screen/film contact. Areas of poor screen/film contact appear as black splotches on the resulting image. Each screen should be marked with an identification number near the left or right edge away from the chest wall. This is accomplished by affixing thin stick-on numbers that do not compromise film screen contact but prevent light from the screen from reaching the film, so that the screen number appears in white on each film. The same number should also be placed on the outside of the cassette. This numbering permits the identification of the particular cassette and its screen should a problem arise, so that artifacts related to the cassette and its screen can be rapidly resolved. Because dust causes artifacts on images and can cause poor screen/film contact, screens should be cleaned at least once a week (more frequently as needed).
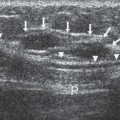
Grid
The grid must be a moving grid. It must function properly and be able to continue to function even when there is firm pressure against it during breast compression. A common cause of grid artifact is poor electrical connection (Fig. 9-1).
Processing
Processing of the film is frequently the weakest link in the imaging chain. Processing involves a fairly sensitive, controlled interaction among the film, the chemicals, and the processor. It has been found that it is usually best to follow the film manufacturer’s guidelines and use combinations of film, chemicals, processor cycle time, and developer temperatures that have been determined to work best. This does not mean that the individual practice cannot discover better combinations, but the recommended processing parameters have usually been carefully arrived at by the manufacturers. It is usually best to consider these components together as a complete system to obtain appropriate image quality, especially in terms of film contrast and speed.
Processor
Ideally the processor is completely dedicated to mammography. If the processor must be used to process other kinds of film, it should be optimized for the mammography film. If the processor must be shared, it is beneficial if all single-emulsion film is directed to the mammography processor, with a separate processor for double-emulsion films, because
the chemistry replenishment rates are determined by the amount of emulsion processed.
the chemistry replenishment rates are determined by the amount of emulsion processed.
Daily processor maintenance includes sensitometry, monitoring of developer temperature, and cleaning of the crossover rollers so that buildup of particulate matter or other material does not scratch or otherwise damage the film. A thorough cleaning should be performed at least monthly, and processor inspection should check for rough rollers, nonuniform transport speed, broken gears, and chemistry replenishment rates.
Control of Processing Artifacts by Proper Maintenance
If the processor is shared, bimonthly cleaning is recommended. A specially manufactured tacky transport roller cleanup film specifically designed to help clean rollers, used daily, may further avoid processing artifacts. Dirt and sludge from the transport rollers clings to the cleanup film, helping to keep the rollers free of dirt in the times between routine cleanings. Because the largest fluctuations in image quality have been found to be associated with processing and the processor, close attention must be paid on a daily basis.
Processor and Processing Chemistry
To maintain consistent quality, the film processor should be optimized for the type of mammographic film being used. If possible, the processor should be used to process these films only, as other films (e.g., larger sizes, double emulsion) can affect the processing characteristics. The choice and concentration of processing chemistries is a vital part of the system. Various chemistries are rarely interchangeable. Film speed, film contrast, and base plus fog are affected by the various types and quantities of chemicals used (Fig. 9-2).
One should be cautious about the use of repackaged chemistries, because there may be a tendency for these not to be as concentrated as the original chemicals from the manufacturer (3). The complex chemical reactions in film processing are beyond the scope of this discussion (see Chapter 8). However, several classes of chemicals are involved. The concentration of hydroquinone affects film contrast and helps determine the ultimate blackening of the film. Phenidone affects the speed of the film and the gray tones of the image. The activity of hydroquinone and phenidone is affected by the pH of the developer solution. Sulfites control oxidation and the concentration of the hydroquinone
so that it is not used up too quickly. A gross measure of the concentration of these chemicals can be obtained by measuring the specific gravity of the solutions, but only direct chemical analysis can provide absolute confirmation of the concentration of each individual component.
so that it is not used up too quickly. A gross measure of the concentration of these chemicals can be obtained by measuring the specific gravity of the solutions, but only direct chemical analysis can provide absolute confirmation of the concentration of each individual component.
Chemical Replenishment Rates
As films are developed, bromide is produced. Bromide is needed to control the development process, but too much results in slower development. The processing parameters are dependent on a balance of bromide washing from the film and slowing development while fresh chemistry is added at the appropriate replenishment rate to maintain the desired concentrations or a seasoned chemistry. As film is processed, the processing chemistries are used up and must be replenished. The proper rate of replenishment of the chemicals is necessary to maintain stable activity of the developer and fixer. The exact rate of replenishment depends on the surface area of the emulsion being processed. Because the processor cannot determine when a dual-emulsion film is being processed, these should be processed separately from single-emulsion mammographic films so that the proper chemistry replenishment rates can be maintained. Some processors track this figure directly. Usually replenishment rates are more crudely calculated using the number and size of the film processed (i.e., the total volume of film being developed per day). It is important to consult with the processor manufacturer to correctly adjust and set up the film processor to establish the appropriate replenishment rates. This will ensure the desired results and the consistency of those results.
Somewhat paradoxically, high film use per day requires lower replenishment rates per sheet of film. Very low film volume (30 to 60 films per day) is difficult to stabilize and flood replenishment is recommended. Flood replenishment requires the use of a starter solution in the replenishment tanks.
Following the initial optimization of film processing, proper replenishment is monitored and established by stable sensitometric results (film speed, film contrast, and base plus fog). The proper concentrations of these chemicals help to control artifacts and ensure that the images will not deteriorate with time.
Processing Time and Temperature
The most important time in the film processing cycle is the time that the film is immersed in the developer chemicals. This and the overall time in the processor should be chosen to optimize the sensitometric performance of the film to meet the diagnostic needs of the radiologist. Different mammography films have different processing characteristics; thus, there is no best processing time or temperature that is appropriate for all mammography film.