Radiation-induced coronary artery disease (RI-CAD) is a significant cardiovascular complication for cancer survivors treated with thoracic radiation therapy (RT). Despite advances in RT techniques, exposure to the heart during treatment remains a critical factor influencing long-term cardiac outcomes, particularly in patients with breast and lung cancer. RI-CAD develops due to radiation-induced endothelial injury, inflammation, and accelerated atherosclerosis, presenting a unique and aggressive disease profile. This review explores the pathophysiology, risk factors, and diagnostic advancements for RI-CAD, emphasizing the role of PET in improving patient outcomes.
Key points
- •
Chest radiation, particularly for breast and lung cancer, triggers a pro-inflammatory response in the coronary arteries, promoting endothelial damage, oxidative stress, and accelerated plaque formation that can present years after treatment.
- •
Patients receiving left-sided thoracic radiotherapy demonstrate higher rates of coronary artery disease (CAD) and related cardiac events due to closer proximity of radiation fields to the heart. The severity and incidence of CAD correlate with the total radiation dose and fractionation schedule, underscoring the need to minimize cardiac exposure whenever possible.
- •
Advanced PET-CT techniques using tracers such as fluorodeoxyglucose (FDG) and sodium fluoride (NaF) enable early detection of vascular inflammation and microcalcifications, providing a window for timely intervention.
- •
18F-NaF PET imaging is particularly effective for visualizing early microcalcifications in coronary plaques–often before they appear on standard imaging–allowing for improved risk stratification and timely management.
- •
Given the potential for late-emerging cardiac effects, extended follow-up and aggressive control of traditional cardiovascular risk factors (e.g., smoking, hyperlipidemia) are essential to mitigate the burden of radiation-induced CAD.
| 3D-CRT | 3-dimensional conformal radiation therapy |
| ACCS | Alavi-Carlsen Calcification Score |
| ACS | Alavi-Carlsen Score |
| CACS | Coronary Artery Calcification Scores |
| CAD | Coronary artery disease |
| CT | computed tomography |
| FDG | fluorodeoxyglucose |
| IMRT | intensity-modulated radiation therapy |
| LAD | left anterior descending artery |
| NaF | sodium fluoride |
| NSCLC | non-small cell lung cancer |
| RI-CAD | Radiation-induced coronary artery disease |
| RI-CAD | radiation-induced coronary artery disease |
| RT | radiation therapy |
Introduction
Coronary artery disease (CAD) is a significant concern in patients who have undergone radiation therapy (RT) for cancers. With advancements in cancer treatments, including RT, the survival rates for patients with cancer have improved. However, these improvements have also brought to light long-term health complications associated with cancer therapies, particularly cardiovascular diseases. Among these, CAD remains one of the leading causes of morbidity and mortality in cancer survivors, especially those treated with RT involving the thoracic region. ,
Coronary artery disease is defined as the narrowing or blockage of the coronary arteries, typically caused by atherosclerosis, which reduces blood flow to the heart muscle. In the context of RT, CAD can develop as a direct result of radiation-induced damage to the coronary arteries, which may include endothelial injury, inflammation, and accelerated atherosclerosis. , The underlying pathology involves a cascade in which radiation exposure triggers a proinflammatory response. This is known as the NF-κB pathway, where radiation leads to the increase in the number of cytokines and adhesion molecules that bring inflammatory cells to the affected region. Patients with RT develop chronic inflammation due to the increase in expression of this NF-κB pathway ( Fig. 1 ). This persistent inflammatory state induces oxidative stress, which can lead to the development of chronic inflammation observed in patients who have received RT. Over time, this inflammatory milieu can accelerate the process of atherosclerosis, potentiating CAD to form. Myocardial infarction is the most common cause of cardiac mortality due to radiation-induced CAD (or RI-CAD). , RI-CAD often presents several years to even decades after treatment and may be more aggressive compared to non-radiation-related CAD.

RT is a treatment modality that uses high doses of irradiation to kill cancer cells and shrink tumors. While effective in treating cancers, RT can also damage surrounding healthy tissues, including the heart and its vasculature. The risk of developing CAD following RT is influenced by several factors, including the total radiation dose, the volume of heart exposed, the dose received by different cardiac substructures, and patient-specific factors such as age, smoking history, and other pre-existing cardiovascular risk factors. ,
The link between RT and cardiovascular diseases was first recognized in patients who received mediastinal irradiation for Hodgkin’s lymphoma in the 1960’s. Early studies documented an increased incidence of myocardial infarctions, pericarditis, and other long-term heart-related complications among these patients. As radiation techniques evolved and were applied to other types of cancers, particularly lung and breast, the scope of these observations expanded. The late effects of RT on the heart, including the development of CAD, became an area of growing concern.
Lung and breast cancers are among the most common malignancies worldwide, and RT is a key component in the treatment of both. Due to the proximity of the heart to the lungs and breast, radiation exposure to the heart is often unavoidable. Advances in RT techniques, such as 3-dimensional conformal RT (3D-CRT), intensity-modulated RT (IMRT), and proton therapy, have aimed to decrease cardiac exposure. Despite these improvements, the risk of developing RI-CAD remains a significant concern, especially as cancer survival rates continue to improve, leading to longer post-treatment follow-up periods. Understanding the pathophysiology, risk factors, and prevention strategies for radiation-induced CAD is crucial for improving the quality-of-life and outcomes for cancer survivors.
Discussion
Association of Chest Radiation for Breast and Lung Cancer with Coronary Artery Disease
RT can lead to an array of adverse cardiac effects, including valvular disease, pericardial disease, cardiomyopathies, and conduction abnormalities. However, coronary artery disease continues to be the most common, with a prevalence of up to 85%. The pathophysiology of this process is a result of the pathogenic communication between oxidatively damaged proteins, DNA, and lipids that are induced by radiation exposure. RI-CAD is thought to differ from traditional atherosclerosis, as radiation can cause both macrovascular changes in large coronary arteries and microvascular damage within the myocardium. This damage progresses slowly, often manifesting years to decades after initial therapy, making long-term surveillance critical. This damage clinically presents with variance, ranging from asymptomatic atherosclerosis through symptomatic angina all the way to an acute myocardial infarction. Factors such as the total radiation dose, radiation fractionation schedule, and proximity of the radiation field to the heart all contribute to the likelihood of developing and severity of RI-CAD. Moreover, traditional cardiovascular risk factors, including smoking, hypertension, and hyperlipidemia, may exacerbate radiation-induced damage, further complicating the clinical course of these patients.
The association between chest radiation, most commonly for breast or lung cancer, and CAD is well-established. Left ventricular segments are particularly sensitive to radiation, suggesting the need to minimize dose exposure. In a study of stage I-II patients with breast cancer treated with RT and subsequently screened via cardiac stress tests and catheterizations, abnormalities were found in 59% of left-side irradiated patients compared to just 8% of right-side patients ( P = .001). 70% of the abnormalities in the left-sided cohort were in the area of the left anterior descending artery (LAD), a coronary segment with strong associations with myocardial infarction and mortality.
Beyond this, many studies have independently described an increased risk for CAD post-RT. A population-based case-control study showed that the risk of a major coronary event increases linearly with the mean heart dose of thoracic RT in women with breast cancer, with risk continuing for at least 20 years after exposure. In another study analyzing 112 patients with stage III non-small cell lung cancer (NSCLC) who received escalated RT (70 – 90 gray or Gy), an independent association was found with increased cardiovascular events, including myocardial infarction with a follow-up period of 8.8 years. This dose-dependent relationship was further solidified by Niska and colleagues, who found that the relative risk for CAD increased by approximately 10% per Gy of mean radiation dose, with additional increases depending on patient age and other risk factors. This is particularly important, as studies of irradiation in both the definitive and the adjuvant settings have shown that higher doses to the heart directly affect survival for patients with thoracic malignancies. ,
Assessing Coronary Artery Disease in Left-Sided Versus Right-Sided Radiation Therapy
Although there is a well-described relationship between mean radiation dose and adverse cardiac events, there is increasing literature demonstrating the significance of radiation location in determining long term RI-CAD. In 2013, Darby and colleagues found that the radiation dose to the heart from left-sided breast cancer was more than double that of right-sided radiation, with a direct linear relationship between radiation dose and coronary events. Another population-based study found that among 20,871 women undergoing RT for breast cancer during the 1970s and 1980s (51% of which had left-sided disease), cardiovascular mortality from any form of cardiovascular disease exceeded 25% after 15 years for left-sided compared to right-sided breast cancer treatment ( P =.014). A more recent study reported in 2016 of 29,102 patients with breast cancer found that the incidence of myocardial ischemia requiring percutaneous coronary intervention (PCI) was higher in women who underwent left-sided radiation (5.5%) versus right-sided (4.5%). However, this risk was particularly high for women with preexisting heart disease. Despite some limitations in study design or confounding comorbidities in patients, early research has shown this association of RT location to be clinically relevant.
The recent 2021 WECARE study took 1,583 women <55 years diagnosed with breast cancer and compared left- and right-sided RT outcomes; the group found the 27.5-year cumulative incidence of CAD to be 10.5% for left-sided RT and 5.8% for the right-sided RT ( P =.010). Even in a multivariate analysis of their data, there was a 2.5 hazard ratio for left-sided vs right-sided RT (95% Cl: 1.3–4.7). The authors were also unable to find any statistically significant baseline factors affecting the increased rate of CAD in left-sided RT, including body mass index and smoking status. A recent review supported these findings, indicating a need for clinical consideration of RT location in patients with complicating comorbidities, as well as further research into the interplay between these risk factors and RT over a longitudinal period.
Similar effects have been reported for patients with left-sided primary lung cancers relative to right-sided tumors. In a population-based analysis of 52,624 NSCLC patients, although cardiac-specific mortality decreased over time as advanced radiation modalities began to be utilized more commonly, there remained a consistent increase in cardiac mortality for patients with left-relative to right-sided tumors, with left-sided laterality independently predicting for cardiac specific mortality. Similarly, in a population-based analysis of 19,692 patients with small cell lung cancer, those with left-sided tumors experienced significantly higher cardiac-specific mortality, with laterality identified as an independent predictor of cardiac mortality.
PET Markers for Assessing Coronary Artery Disease
Surveillance for RI-CAD is clearly critical, especially in patients who may have prior risk factors. Current imaging modalities, such as computed tomography (CT) coronary angiography and PET play essential roles in identifying early vascular and myocardial changes. CT angiography allows for non-invasive visualization of the coronary arteries, enabling the detection of calcification, stenosis, and plaque formation. It is particularly useful for identifying subclinical atherosclerosis and for assessing overall coronary artery calcium (CAC) scores, which have been found to correlate with future cardiovascular risk in this population.
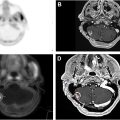
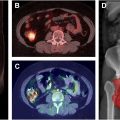
PET, particularly when combined with CT (PET-CT), offers additional functional insight. PET can assess myocardial perfusion and viability, identifying areas of ischemia or infarction that may not be visible on CT alone. 18F-Fluorodeoxyglucose (FDG) is a widely used PET tracer for identifying inflammation in most organs in the body. Because activated inflammatory cells have high glycolytic activity, FDG-PET/CT imaging can be used to detect inflammation within the large or major arteries such as the aorta and the myocardium, providing an early marker of radiation-induced atherosclerosis before structural changes become apparent, and visible on traditional imaging modalities like CT ( Fig. 2 ).


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree